To characterize mechanical ventilation-associated pneumonia (MVAP).
MethodThis is an observational descriptive study to characterize MVAP in 61 ventilated patients admitted in the intensive care units of the Hermanos Ameijeiras hospital during 2011. This study also aimed to isolate the bacteria causing MVAP and characterize their resistance to antibiotics.
Results51 (83.60%) patients presented pulmonary infiltrates and 35 (50.81%) presented a clinical score ≥ 6 according to the Clinical Pulmonary Infection Score. Acinetobacter baumannii and Pseudomonas aeruginosa were the most frequently isolated microorganisms from patients with MVAP. Both microorganisms showed a high resistance to antibiotics. Carbapenems were the most frequent used antimicrobial therapeutic agents; elective antibiotic combinations were directed against both bacterial wall structure and nucleic acid synthesis.
ConclusionPatients with MVAP identified during the studied period showed similar frequency to those reported in medical literature. Thus, this study corroborated that this is still a relevant medical problem in this hospital. Acinetobacter baumannii and Pseudomonas aeruginosa were the most frequently isolated microorganisms from patients with MVAP. Antimicrobial treatment, empirical or not, are still the main risk factors for the development of multidrug-resistant strains of bacteria. The rate of resistance to antibiotics of Acinetobacter baumannii and Pseudomonas aeruginosa strains isolated from patients with MVAP was higher than those isolated from infected patients without MAVP. Tigecycline and colistin were the only antibiotics fully effective against Acinetobacter baumannii strains isolated in 2011 from patients with MVAP; against Pseudomonas aeruginosa strains, only colistin was fully effective.
Mechanical ventilation-associated pneumonia (MVAP) accounts for approximately 80% of nosocomial pneumonia episodes. This term is applicable only in intubated or tracheostomized patients who develop pneumonia under mechanical ventilation.1 The mechanical ventilation procedure was first used in animals in 1543 by Andreas Vesalius. Galen was the first physician to describe its use in humans.2
MVAP is one of the most common infections in the intensive care units (ICUs), increasing the length of stay of patients in these units, the cost of the treatment, and the risk of death.3 The etiology of MVAP depends on multiple factors such as time of ventilation, prior administration of antibiotics, presence of chronic obstructive pulmonary disease, coma, and local factors.4 Various microbial agents such as non-fermentative Gram-negative multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa have been described, over the last decades, as agents that cause this type of mechanical ventilation-associated infection.5
The main mechanism in the pathogenesis of MVAP is based on the repeated micro-aspiration of microorganisms that colonize the upper airways through the space between the endotracheal tube cuff and trachea wall. The origin of these microorganisms varies from the patient's endogenous microbiota to exogenous sources, mainly the hands of healthcare workers or contaminated nebulizers.6–8
ObjectivesDue to the importance of MVAP and the consequences for patient survival and healthcare costs, a study to characterize MVAP is performed in the Hermanos Ameijeiras Hospital (HAH) annually. This article discusses the data collected in the year 2011. Thus, the main objectives of this study were to characterize MVAP, to isolate antimicrobial agents in tracheal fluid samples, to identify most frequent causative microorganisms, and to calculate the resistance rate to antibiotics of the most frequently isolated microorganisms responsible for MVAP in ICU patients.
Materials and methodsGeneral information of hospitalThe HAH is located in Havana, Cuba. It has 625 beds, 15 specialized services, and 12 surgical units, as well as three ICUs. The institution provides care to the adult population exclusively.
Universe of patientsAll ventilated patients in the ICU was considered as the universe of the study.
Criteria for patient inclusionAll ventilated patients admitted in the ICU during 2011 with available tracheal aspirates for microbiological testing were included.
Statistical analysisClinical data were gathered in a data base model (Statistical Package for Social Sciences – SPSS.17) designed by the Microbiology Department. Data processing was performed using the Excel software and the statistical software Statgraphics Plus version 5.0 (2000). Pearson's chi-squared test was used to analyze the association among qualitative variables, and Student's t-test was performed to compare the number of isolated strains and the resistance to antibiotics. The significance level was set at 0.05.
Criteria for the clinical diagnosis of MVAPClinical diagnosis of MVAP was performed following the Clinical Pulmonary Infection Score (CPIS).9 Patients with clinical scores ≥ 6 were considered positive for MVAP.
Sample processing and microorganism identificationThe processing of the samples for microbiological diagnosis was performed according to the norms and procedures of the Centers for Disease Control (CDC).10 The diagnosis of the microorganisms was performed using the Vitek 2 Compact automated equipment (bioMériux – France).
This is an antimicrobial resistance study of Acinetobacter baumannii and Pseudomonas aeruginosa strains isolated from patients with or without MAVP.
In order to test the antimicrobial resistance to antibiotics, the Vitek 2 Compact automated equipment (bioMériux – France) was used with AST N87 cards. The antimicrobials assessed to compare the resistance of the Acinetobacter baumannii and Pseudomonas aeruginosa strains isolated from patients with and without MVAP were: amikacin (10μg), ampicillin (10μg), ampicillin-sulbactam (10μg-10μg), aztroenam (30μg), cefepime (30μg), cefoxitine (30μg), ceftazidime (30μg), ceftriaxone (30μg), ciprofloxacin (5μg), colistin (10μg), gentamicin (10μg), imipenem (10μg), meropenem (30μg), nalidixic acid (30μg), and tigecycline (15μg). The strains ATCC BAA-747 (Acinetobacter baumannii) and ATCC 27853 (Pseudomonas aeruginosa) were used as reference materials.
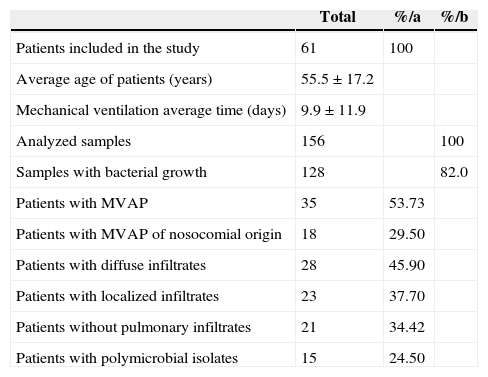
ResultsFrom the 61 patients included in this research, 28 (45.9%) and 23 (37.7%) had diffuse and localized infiltrates, respectively, which are relevant signs of MVAP. 35 patients (53.73%) showed an infiltrate score ≥ 6 according to CPIS9 and three (4.90%) were diagnosed post-ventilation. Polymicrobial isolates were detected in 15 patients (24.59%). Regarding the studied samples, a total of 156 tracheal aspirates were tested. Bacterial growth (> 104 ufc/mL) was observed in 128 samples (82.05%). The average ventilation time was 9.9 ± 11.9 days and the average age of the studied patients was 55.5 ± 17.2 years old (Table 1).
Results of the clinical-microbiological characterization of MVAP.
| Total | %/a | %/b | |
|---|---|---|---|
| Patients included in the study | 61 | 100 | |
| Average age of patients (years) | 55.5 ± 17.2 | ||
| Mechanical ventilation average time (days) | 9.9 ± 11.9 | ||
| Analyzed samples | 156 | 100 | |
| Samples with bacterial growth | 128 | 82.0 | |
| Patients with MVAP | 35 | 53.73 | |
| Patients with MVAP of nosocomial origin | 18 | 29.50 | |
| Patients with diffuse infiltrates | 28 | 45.90 | |
| Patients with localized infiltrates | 23 | 37.70 | |
| Patients without pulmonary infiltrates | 21 | 34.42 | |
| Patients with polymicrobial isolates | 15 | 24.50 |
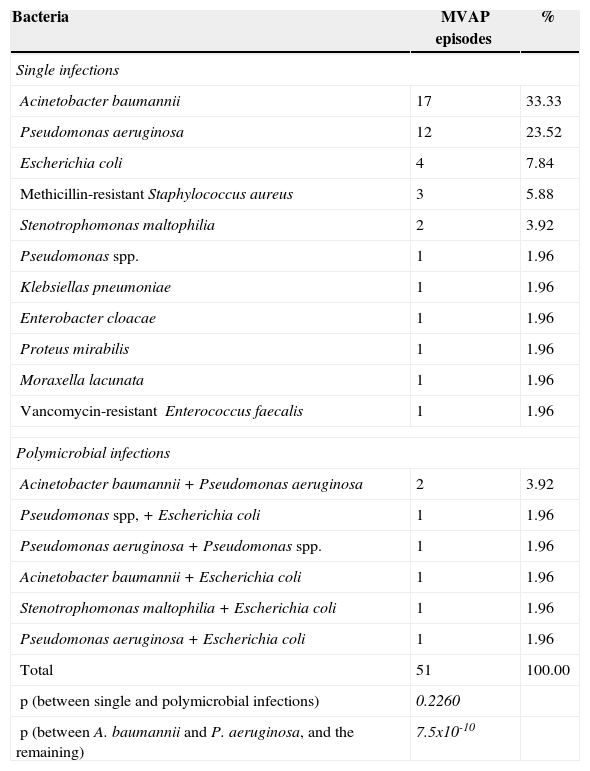
The microorganisms isolated from this universe of patients with MVAP were: Acinetobacter baumannii, Pseudomonas aeruginosa, Escherichia coli, meticillin-resistant Staphylococcus aureus, Stenotrophomonas maltophilia, Pseudomonas spp., Klebsiella pneumoniae, Enterobacter cloacae, Proteus mirabilis, Moraxella lacunata, and vancomycin-resistant Enterococcus faecalis. The polimicrobial infections observed were: Acinetobacter baumannii + Pseudomonas aeruginosa, Pseudomonas spp. + Escherichia coli, Pseudomonas aeruginosa + Pseudomonas spp., Acinetobacter baumannii + Escherichia coli, Stenotrophomonas maltophilia + Escherichia coli, and Pseudomonas aeruginosa + Escherichia coli. Table 2 shows the number and proportion of each isolate in detail. The proportion of infections with Acinetobacter baumannii and Pseudomonas aeruginosa was significantly higher than the other isolated bacteria (p = 7.5 x 10−10).
Proportions of microorganisms isolated from patients with MVAP (n = 51).
| Bacteria | MVAP episodes | % |
|---|---|---|
| Single infections | ||
| Acinetobacter baumannii | 17 | 33.33 |
| Pseudomonas aeruginosa | 12 | 23.52 |
| Escherichia coli | 4 | 7.84 |
| Methicillin-resistant Staphylococcus aureus | 3 | 5.88 |
| Stenotrophomonas maltophilia | 2 | 3.92 |
| Pseudomonas spp. | 1 | 1.96 |
| Klebsiellas pneumoniae | 1 | 1.96 |
| Enterobacter cloacae | 1 | 1.96 |
| Proteus mirabilis | 1 | 1.96 |
| Moraxella lacunata | 1 | 1.96 |
| Vancomycin-resistant Enterococcus faecalis | 1 | 1.96 |
| Polymicrobial infections | ||
| Acinetobacter baumannii + Pseudomonas aeruginosa | 2 | 3.92 |
| Pseudomonas spp, + Escherichia coli | 1 | 1.96 |
| Pseudomonas aeruginosa + Pseudomonas spp. | 1 | 1.96 |
| Acinetobacter baumannii + Escherichia coli | 1 | 1.96 |
| Stenotrophomonas maltophilia + Escherichia coli | 1 | 1.96 |
| Pseudomonas aeruginosa + Escherichia coli | 1 | 1.96 |
| Total | 51 | 100.00 |
| p (between single and polymicrobial infections) | 0.2260 | |
| p (between A. baumannii and P. aeruginosa, and the remaining) | 7.5x10-10 | |
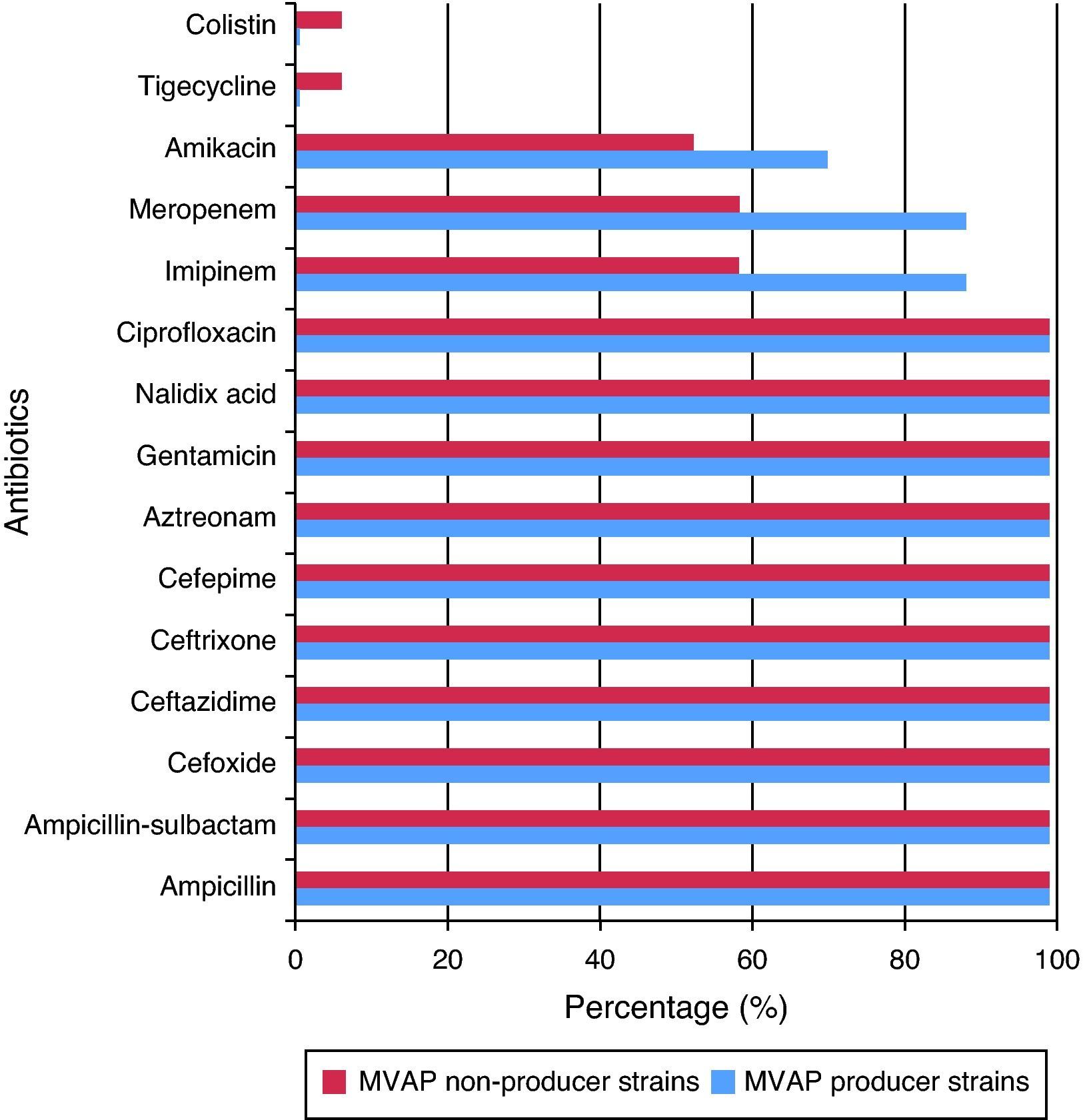
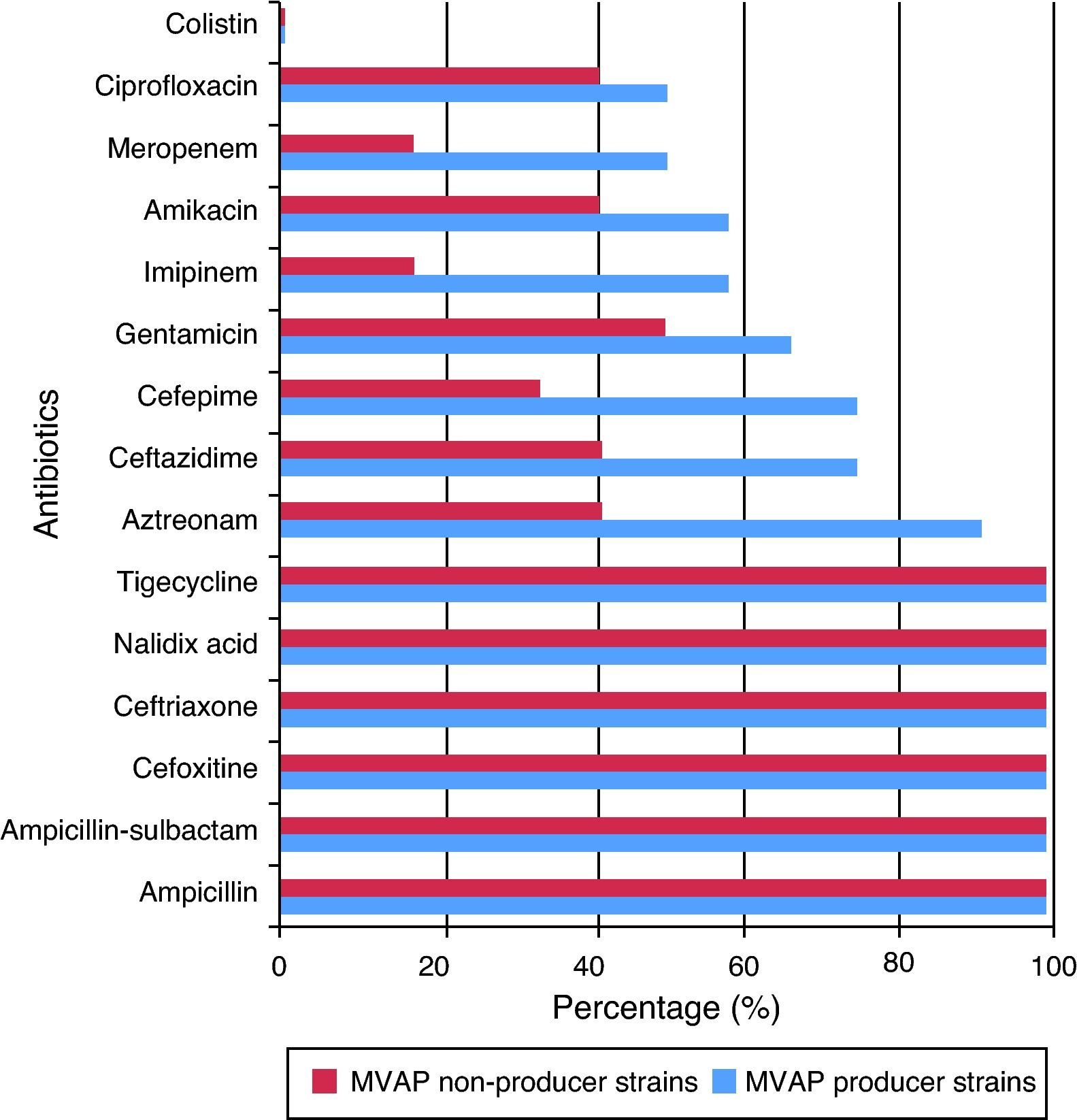
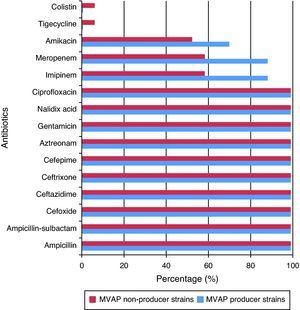
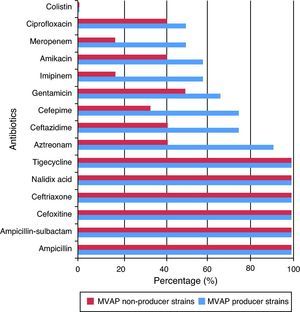
Resistance rates to 15 different antimicrobials of the Acinetobacter baumanni and Pseudomonas aeruginosa strains isolated from patients with and without MVAP are shown in Figs. 1 and 2, respectively. Resistance rates to antimicrobials among isolates of Acinetobacter baumannii (n = 17) were only different with regard to imipenem, meropenen, and amikacin. However, no statistically significant differences were detected in any of these cases (p = 0.23). In the case of the Pseudomonas aeruginosa isolates, resistance rates to antimicrobials of the MVAP isolates were higher for ceftazidime, cefepime, aztreonamm imipinem, meropenem, amikacin, gentamicin, and ciprofloxacin. However, significant difference was only observed for aztreonam (p = 0.03).
MVAP is more frequent and severe exhibiting a high mortality.11 The clinical diagnosis of this disease is established by the emergence of new or progressive infiltrates on chest radiography, followed by two or three additional criteria recommended by the CPIS since 1991.9 But today, the importance of traditional and automated microbiology, as a fundamental tool for the diagnosis of these infections, is emphasized, particularly when initiating or changing antimicrobial treatment and assessing the effectiveness of bacterial quantification.12
According to literature, 50% to 60% of patients with mechanical ventilation for over 48h in ICUs developed pneumonia, and approximately 52% of these are of nosocomial origin. The mortality attributed to this type of infection ranged between 24% to 76% in mechanically ventilated patients for more than 48h.13,14 These findings are consistent with those found in this study, since 53.73% of the patients admitted for an average of 9.9 ± 14.1 days to the ICU of the HAH developed MVAP, and out of those, 51.4% had a nosocomial origin. Nevertheless, these results contrast with those of the national surveys conducted by Labaut et al., who have demonstrated lower rates of the MVAP than those reported in the HAH,15 despite sharing the same health system and geographical area.
The etiology of MVAP was described several years ago, and it varies according to the characteristics of the geographic area and ICU, and the ventilation time of the patients.14 Gram-negative bacteria, such as Pseudomonas aeruginosa, Acinetobacter spp. and Enterobacteriaceae, represent from 55% to 85% of MVAP cases; Staphylococcus aureus, from 20% to 30%; and 40% to 60% are polymicrobial infections.16
In a recent study performed by Ortiz et al., Pseudomonas aeruginosa, Klebsiella pneumoniae and Staphylococcus aureus meticillin-sensitive were described as the bacteria associated to MVAP in 39 ICUs in Colombia.17. Ruiz et al., in another Latin American study, identified methicillin-resistant Staphylococcus aureus and polymicrobial infections18 as leading causes of pneumonia in ventilated patients. The MVAP consensus conducted in 2011 established that the microorganisms that most commonly affect patients undergoing mechanical ventilation in the ICU were: methicillin-sensitive Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, Pseudomonas spp., Acinetobacter spp., and Enterobacteriaceae.19
Most of the microorganisms reported as causal agents of MVAP are consistent with those found in the present study. In this study, 11 different bacteria were isolated from patients with MVAP. Within these patients, the prevailing bacteria (56.82%) were Acinetobacter baumannii (33.33%) and Pseudomonas aeruginosa (23.52%). These results are consistent with those reported in 2002 by González et al. where Acinetobacter baumannii and Pseudomonas aeruginosa were the main causative microorganisms of MVAP in this hospital.20 As mentioned above, the proportion of infections by these two microorganisms was statistically different than the rest of the isolated bacteria from patients with MVAP.
As was found in this study, Acinetobacter baumannii has probably been the most frequently isolated bacterium from patients with MVAP around the world. Pneumonia caused by this microorganism is highly related with previous use of antimicrobials, and contamination of ventilation equipment and hands of health workers.21,22 It is the authors’ opinion that antimicrobial treatment, empiric or not, is the principal risk factor for the development of multidrug-resistant strains of this bacterium, and thus of the high proportion of contamination in patients with MVAP.
The relative high proportion of infection with Pseudomonas aeruginosa is in agreement with that reported by others.17,23,24 This finding has been associated with the possibility that this microorganism penetrates the lower respiratory tract of the ventilated patients employing two ways: endogenous and exogenous,25 and also by the special tropism of Pseudomonas aeruginosa to the tracheal epithelium, as suggested by Niederman et al.26
The other isolated bacteria (Escherichia coli, methicillin-resistant Staphylococcus aureus, Stenotrophomonas maltophilia, Pseudomonas spp., Klebsiella pneumoniae, Enterobacter cloacae, Proteus mirabilis, Moraxella lacunat, and vancomycin-resistant Enterococcus faecalis) represented only 29.4% of the infections. However, around 13.72% of infections were polymicrobial. Therefore, it should be mentioned that infections of polymicrobial origin also play a role in the onset of MVAP, which is also coincident with other reports.16 In that sense, a behavioral association of Escherichia coli with different species of bacteria was observed as cause of the MVAP, a phenomenon that was not found in the examined literature. Several authors have demonstrated the existence of mixed infections, but they did not describe the microorganisms in detail.14–18 Perhaps the association of Escherichia coli with other microorganisms causing MVAP is associated with the invasive mechanisms and high prevalence of this enterobacterium in the digestive tract as part of the intestinal microbiota. In that sense, the use of nasogastric probes may favor upward migration, colonization, and infection from the lower airways.
Bacterial resistance is one of the most important problems that affect hospitals, especially ICUs. Antimicrobial therapy becomes more limited every day due to bacterial resistance mechanisms created by the indiscriminate use of these drugs, which could turn medicine into an era where there were no antimicrobials to treat bacterial infections.27
According to the Infectious Diseases Society of America and the American Thoracic Society, empiric antimicrobial treatment is linked with the emergence of multidrug-resistant strains, indicating that this practice must stop and be replaced by rapid microbiological tests, which will play a crucial role in the diagnosis of MVAP and in the establishment of appropriate therapies to prevent the emergence of multidrug-resistant strains and worsening of infection.28 Ruiz C et al., confirmed that empirical treatment induced the emergence of resistant strains causing MVAP in their hospital;18 in a retrospective review conducted in 2002, the empirical antimicrobial treatment in ventilated patients of the HAH was evidenced,19 which could explain the high resistance of the bacteria currently encountered.
Acinetobacter baumannii is considered one of the leading bacteria for the production of mechanisms that induce antimicrobial resistance. Meropenem and imipenem in particular, are used in the treatment of infections caused by Acinetobacter baumannii strains because they have shown higher in vitro activities than other antimicrobial agents. Nevertheless, carbapenem resistance in these species is increasing significantly; it is a sentinel sign for the emergence of multidrug-resistant strains.29 However, due to several reasons, antimicrobial resistance results cannot be extrapolated under any circumstances. For instance, a study conducted in 2005, which compared strains of Acinetobacter baumannii isolated in a hospital in Madrid, Spain, with those isolated in a hospital in Hong Kong, China,30 demonstrated that over 90% of the isolates obtained from both hospitals were sensitive to imipenem. This finding is in contrast with the results of the present study. In addition, an investigation performed at the HAH31 also demonstrated that resistance of Acinetobacter baumannii strains to imipenem increased from 2002 to 2010 by over 80%, and continued increasing in 2011 according to the results of this study.
A significant increase was also observed in imipenem resistance of the Acinetobacter baumannii strains isolated from the MVAP-patients of the HAH32 in comparison with the resistance to this antibiotic of strains isolated in a study performed in Europe and Asia.30
Some upregulation mechanisms of chromosomally mediated efflux pumps have been described as causes for the resistance to tigecycline by other researchers.33 However, tigecycline has been reported as an effective antimicrobial agent against resistant species of Acinetobacter baumannii.34 Thus, it is used as an effective therapeutic measure in many cases of serious infection with strains of this microorganism. No tigecycline-resistant strains of Acinetobacter baumannii were found in patients with MVAP in this study. Interestingly, one resistant strain was isolated from a patient with infection but without MVAP.
Colistin is another option for treating infections by this bacterium. It causes alterations in the bacterial cell membrane, increasing permeability and leading to cell death. Its effect is dose-dependent, but some cases of resistant Acinetobacter baumannii strains have already been reported,30,34 possibly as a result of alterations in the outer cell membrane or efflux pump mechanisms. However, similarly to tigecycline, no colistin-resistant strains of Acinetobacter baumannii were detected in the samples of patients with MVAP.
As a final point that is very interesting and not easily found in the literature, regarding meropenem, imipenem, and amikacin, a higher percentage of resistance of MVAP-associated strains with respect to non-MVAP-associated strains of Acientobacter baumannii was found in this study, so it could be inferred that resistance to these drugs is an important risk factor for developing pneumonia by Acinetobacter baumannii in this hospital.
Regarding Pseudomonas aeruginosa, it primarily affects patients with impaired local or general defense mechanisms against infections; hence, it may be considered as an opportunistic pathogen. It is the main cause of nosocomial infections acquired in ICU, because Pseudomonas aeruginosa is naturally resistant to many commonly used antimicrobials in clinical practice. In this study, the high resistance of the Pseudomonas aeruginosa strains isolated from patients with MVAP was also confirmed. Colistin was the only antimicrobial completely effective against this bacterium. In that sense, one could speculate that this resistance may be due to the permeability barrier provided by its outer membrane lipopolisaccharide, efflux pumps, and plasmid-mediated antimicrobial resistance, among other factors. Of interest, although resistance of the strains isolated from patients with MVAP was higher to aztreonam, ceftazidime, cefepime, gentamicin, imipenem, amikacin, meropenem, and ciprofloxacin, significant differences were only observed with aztreonam.
One aspect that has been widely discussed in the literature is the desirability of combined antibiotic therapy over monotherapy for serious infections by Pseudomonas aeruginosa.31 Advantages of the combined therapy are the increase in the possibility that the pathogen is sensitive to at least one of the two antimicrobials prescribed, the possibility of preventing the development of resistance, and the synergistic effect of the combination. However, the hypothesis that the combined antibiotic therapy may increase the risk of toxicity of the treatment, increase costs, and increase the risk of superinfection, cannot be discarded. However, from the analysis of the results of this study, the combination of any antimicrobial assessed here against Pseudomonas aeruginosa is not recommended. The single antibiotic of choice should be colistin.
In summary, MVAP detected during the studied period showed a similar frequency to that reported in medical literature. Acinetobacter baumannii and Pseudomonas aeruginosa were the main microorganisms that affected patients with MVAP. Antimicrobial treatments, empiric or not, are still the main risk factors for the development of multidrug-resistant strains. Resistance to antibiotics of Acinetobacter baumannii and Pseudomonas aeruginosa strains isolated from patients with MVAP was higher than in those isolated from patients with infection but without MAVP. Tigecycline and colistin were the only antibiotics fully effective against of Acinetobacter baumannii strains. Colistin was the only antibiotic fully effective against Pseudomonas aeruginosa strains.
Conflict of interestAll authors declare to have not conflict of interest.
The authors would like to thank BSc. Beatriz Gago Rodríguez for her assistance in the manuscript edition.
This study was supported by the Hermanos Ameijeiras Hospital, which receives funding exclusively from the Cuban Government. Ethical approval for this study was required.