In Brazil, though Antiretroviral Therapy (ART) is available to all, the benefits may not be experienced uniformly. We projected Life Expectancy (LE) for People Living with HIV (PLHIV) in care as currently observed and estimated the impact of guideline-concordant care.
MethodsUsing a microsimulation model, we projected LE for a cohort of PLHIV and for four population groups: cisgender Men who have Sex with Men (MSM), cisgender Men who have Sex with Women (MSW), Cisgender Women (CGW), and Transgender Women (TGW). Cohort data from Evandro Chagas National Institute of Infectious Diseases/Oswaldo Cruz Foundation (INI/Fiocruz) informed model parameters. We modeled five scenarios: 1) Current care: ART initiation, adherence, and retention in care as currently observed, 2) Guideline-concordant care: immediate ART initiation, full adherence to treatment, and consistent retention in care, 3) Immediate ART initiation with observed adherence to treatment and retention in care, 4) Full adherence to treatment with observed timing of ART initiation and retention in care, and 5) Consistent retention in care with observed timing of ART initiation and adherence.
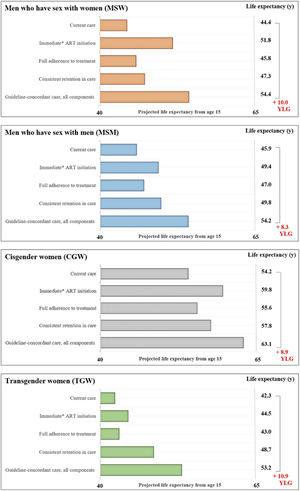
ResultsWith current care, LE from age 15 would be 45.9, 44.4, 54.2, and 42.3 years, for MSM, MSW, CGW, and TGW. With guideline-concordant care, LE would be 54.2, 54.4, 63.1, and 53.2 years, for MSM, MSW, CGW and TGW, with TGW experiencing the greatest potential increase in LE (10.9 years). When investigating the components of care separately, MSW and CGW would gain most LE with immediate ART initiation, whereas for MSM and TGW consistent retention in care would be most impactful.
ConclusionsIn settings like INI/Fiocruz, MSW and CGW would benefit most from interventions focused on earlier diagnosis and linkage to care, whereas TGW and MSM would benefit from interventions to sustain engagement in care. Assessment of the HIV care continuum for specific populations should inform care priorities.
Life Expectancy (LE) is a valuable measure of the overall health of a population. Estimating LE for different population groups can help assess the impact of current policies and inform the planning of additional programs focused on LE improvement, including among People Living with HIV (PLHIV).1 Antiretroviral Therapy (ART) has had a dramatic impact in reducing morbidity and mortality in PLHIV.2 Due to ART, LE in PLHIV receiving guideline-concordant care approaches that of general populations.3 In Latin America and the Caribbean, LE for PLHIV has dramatically increased from 31.0-years in 2003‒2008 to 69.5-years in 2013‒2017.4
However, the benefits of improvements in HIV care are not observed uniformly across vulnerable population groups at highest risk for HIV acquisition. Stigma, prejudice, and discrimination experienced by those with marginalized identities are key determinants of health.5 Recent evidence shows that transgender people have experienced increased mortality compared to cisgender people, with lack of social acceptance, tobacco use, and HIV infection contributing to their increased risk.6
In Brazil, although HIV testing, care and treatment are provided free-of-charge to individuals through the public health system (Sistema Único de Saúde, SUS), disparities by gender and sexual orientation are evident throughout the continuum of care. Studies that compared cisgender women to cisgender men found that women were less likely have initiated ART and to be retained in care,7 and were also at increased odds of virologic failure.8 Another nation-wide study that included 269,076 adults (≥15-years) living with HIV who had started ART from 2006 to 2015 found significantly higher mortality among cisgender men compared to cisgender women.9
These observations suggest that LE in PLHIV may differ substantially by gender and sexual orientation. We sought to quantify disparities in LE in PLHIV in Brazil for four gender and sexual orientation stratified population groups: cisgender Men who have Sex with Men (MSM), cisgender Men who have Sex with Women (MSW), Cisgender Women (CGW), and Transgender Women (TGW). We projected LE with HIV care as currently observed for each group and compared this to what could be achieved with guideline-concordant care.
MethodsAnalytic overviewWe used the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) model to project LE for an overall cohort of PLHIV and for four gender and sexual orientation stratified population groups (MSM, MSW, CGW, and TGW). We considered five scenarios: 1) Current care; 2) Guideline-concordant care; 3) Guideline-concordant timing of ART initiation, with observed adherence to treatment and retention in care; 4) Guideline-concordant adherence to treatment, with observed timing of ART initiation and retention in care; 5) Guideline-concordant retention in care with observed timing of ART initiation and adherence. Current care implies timing of treatment initiation, adherence to treatment, and retention in care as currently observed for these population groups in Brazil.10-12 Brazilian guidelines for care and treatment of PLHIV recommend initiating treatment at the time of diagnosis.13 As a conservative assumption for guideline-concordant initiation of treatment, we assumed that, on average, time from HIV diagnosis to treatment initiation was 5.5-months. Guideline-concordant adherence assumes 95% adherence to treatment, and guideline-concordant retention in care assumes that no one is lost to follow up after entering care, meaning that HIV care is not interrupted despite potential transfers between health services. Because HIV incidence and, therefore, age at HIV acquisition, differs according to gender and sexual orientation,14,15 we did not model individuals from age of HIV acquisition. Instead, to ensure comparability across cohorts and scenarios, we assumed a uniform age at model start and calculated LE from this age. To fully capture HIV incidence and because of the increasing number of infections reported in recent years, particularly among men,16 we defined the age at model start as 15-years. For all simulated cohorts, at model start, individuals are 15-years of age and do not have HIV.
CEPAC model structureCEPAC is a state-transition microsimulation model of HIV prevention and treatment.17,18 The model generates individuals one at a time according to user-defined demographic and clinical characteristics and follows these individuals through monthly cycles among health states until death. The HIV-specific health states are defined by CD4 count, HIV RNA level, ART use, and history of opportunistic infection. The model tracks clinical events and the amount of time in different health states of HIV care, such as uninfected, undiagnosed HIV infection, diagnosed HIV infection and engaged in care, and diagnosed HIV infection and out of care. Once the entire cohort has passed through the model, overall outcome measures such as average LE from model start are recorded.
In the model, following infection with HIV and prior to diagnosis and ART initiation, individuals experience a monthly decline in CD4 count. Age and sex-stratified mortality risks are dependent on CD4 count and history of opportunistic infections. Individuals are subject to HIV testing at specified monthly probabilities.
If individuals are diagnosed with HIV and linked to care, they initiate ART, which decreases mortality through viral suppression and CD4 count increase, with efficacy mediated by adherence to medication.19 Individuals with a high adherence level tend to have higher likelihoods of virologic suppression and retention in care. People with a low adherence level have a lower likelihood of experiencing virologic suppression, therefore resulting in continued HIV replication and decline in health status, and a higher likelihood of loss to follow-up. Additionally, individuals who leave care experience monthly probabilities of returning to care.
Full details of the model are available at https://mpec.massgeneral.org/cepac-model/.
Model inputsModel parameters for cohort characteristics, treatment use and adherence, and natural history were derived from the HIV Clinical Cohort at the Instituto Nacional de Infectologia Evandro Chagas (INI/Fiocruz), and from published literature from Brazil.10-12 INI/Fiocruz is a public research and healthcare institution in Rio de Janeiro, Brazil, and is one of Brazil's largest reference centers for HIV research and treatment. It has provided care to PLHIV in the Rio de Janeiro metropolitan area since 1986.
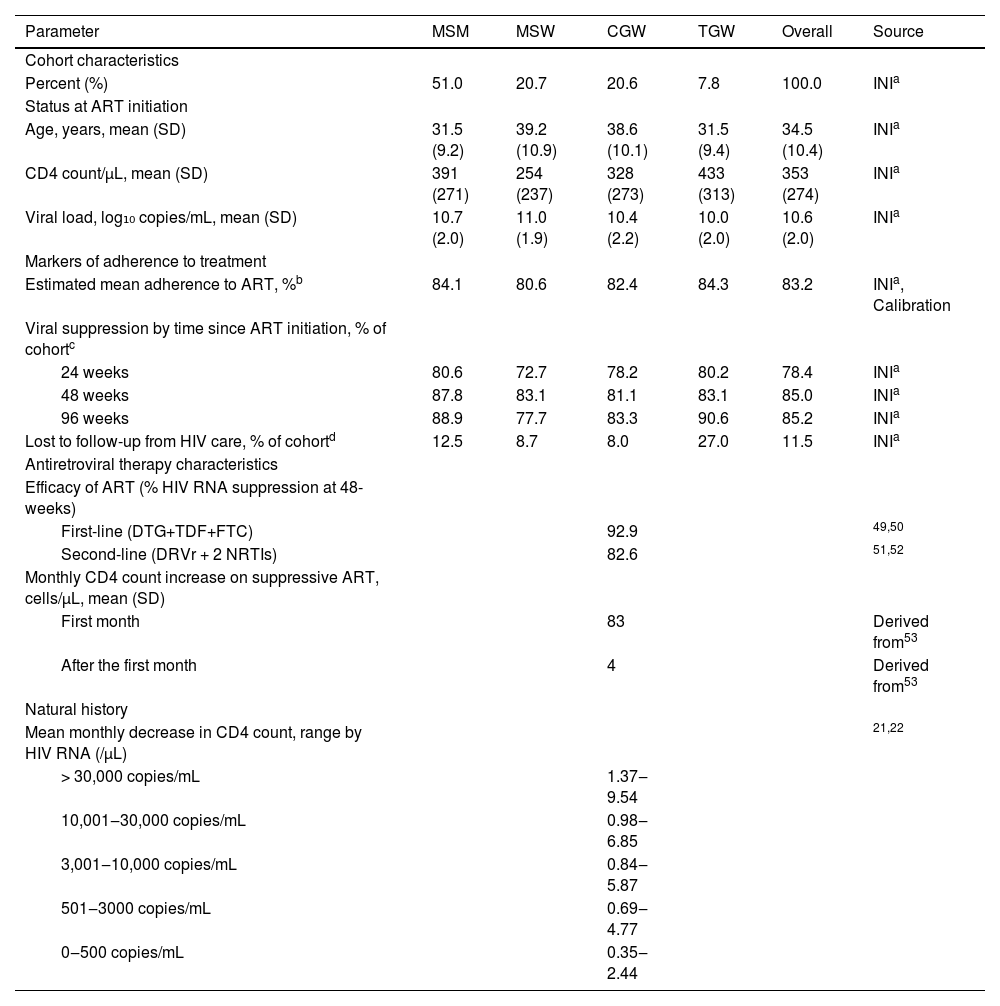
Cohort characteristicsCohort characteristics for each cohort are based on data from PLHIV enrolled and starting ART at the HIV Clinical Cohort between January 1, 2010 and December 31, 2019. The overall cohort is described by the weighted averages of the four gender and sexual orientation-stratified groups as follows: 51.0% MSM, 20.7% MSW, 20.6% CGW, and 7.8% TGW (Table 1). Additionally, to characterize participants’ clinical profile at the start of ART, we selected CD4 counts recorded closest to the participant's ART initiation (365 days before and up to 30 days after).
Key input parameters in the modeling of life expectancy among people with HIV overall and by gender and sexual orientation population groups: cisgender Men who have Sex with Men (MSM), cisgender Men who have Sex with Women (MSW), Cisgender Women (CGW), and Transgender Women (TGW).
| Parameter | MSM | MSW | CGW | TGW | Overall | Source |
|---|---|---|---|---|---|---|
| Cohort characteristics | ||||||
| Percent (%) | 51.0 | 20.7 | 20.6 | 7.8 | 100.0 | INIa |
| Status at ART initiation | ||||||
| Age, years, mean (SD) | 31.5 (9.2) | 39.2 (10.9) | 38.6 (10.1) | 31.5 (9.4) | 34.5 (10.4) | INIa |
| CD4 count/µL, mean (SD) | 391 (271) | 254 (237) | 328 (273) | 433 (313) | 353 (274) | INIa |
| Viral load, log₁₀ copies/mL, mean (SD) | 10.7 (2.0) | 11.0 (1.9) | 10.4 (2.2) | 10.0 (2.0) | 10.6 (2.0) | INIa |
| Markers of adherence to treatment | ||||||
| Estimated mean adherence to ART, %b | 84.1 | 80.6 | 82.4 | 84.3 | 83.2 | INIa, Calibration |
| Viral suppression by time since ART initiation, % of cohortc | ||||||
| 24 weeks | 80.6 | 72.7 | 78.2 | 80.2 | 78.4 | INIa |
| 48 weeks | 87.8 | 83.1 | 81.1 | 83.1 | 85.0 | INIa |
| 96 weeks | 88.9 | 77.7 | 83.3 | 90.6 | 85.2 | INIa |
| Lost to follow-up from HIV care, % of cohortd | 12.5 | 8.7 | 8.0 | 27.0 | 11.5 | INIa |
| Antiretroviral therapy characteristics | ||||||
| Efficacy of ART (% HIV RNA suppression at 48-weeks) | ||||||
| First-line (DTG+TDF+FTC) | 92.9 | 49,50 | ||||
| Second-line (DRVr + 2 NRTIs) | 82.6 | 51,52 | ||||
| Monthly CD4 count increase on suppressive ART, cells/µL, mean (SD) | ||||||
| First month | 83 | Derived from53 | ||||
| After the first month | 4 | Derived from53 | ||||
| Natural history | ||||||
| Mean monthly decrease in CD4 count, range by HIV RNA (/µL) | 21,22 | |||||
| > 30,000 copies/mL | 1.37‒9.54 | |||||
| 10,001‒30,000 copies/mL | 0.98‒6.85 | |||||
| 3,001‒10,000 copies/mL | 0.84‒5.87 | |||||
| 501‒3000 copies/mL | 0.69‒4.77 | |||||
| 0‒500 copies/mL | 0.35‒2.44 | |||||
Note: Mean age at HIV infection was 28.2, 32.0, 28.9, and 33.7 years for MSM, MSW, CGW, and TGW. At ART initiation, 25.1%, 51.9%, 37.8%, and 27.3% of MSM, MSW, CGW, and TGW had CD4 counts < 200 µL.
Abbreviations: PLHIV, People with HIV; MSM, Men who have Sex with Men; MSW, Men who have Sex with Women; CGW, Cisgender Women; TGW, Transgender Women; ART, Antiretroviral Therapy; DTG, Dolutegravir; TDF, Tenofovir Disoproxil Fumarate; FTC, Emtricitabine; DRV/r, Ritonavir-boosted darunavir; NRTI, Nucleoside and nucleotide analogue Reverse Transcriptase Inhibitors.
Derived from the HIV Clinical Cohort at INI/FIOCRUZ among those enrolled between 2010‒2019 with follow-up data until the end of 2020.
Adherence estimates are a result of model calibration, using viral suppression and lost to follow-up data. They represent the mean adherence value experienced by any given individual in that group.
We calibrated monthly HIV incidence so that the modeled age of HIV acquisition aligns with that expected for each group based on the observed age at ART initiation (Table 1) and the natural history of CD4 decline.20-22 We back-calculated the average age at HIV infection from the ages and CD4 counts observed at presentation to care, using a rate of decline of 4.17 cells/uL/mth (50.4 cells/uL/year), derived from Concerted Action on Seroconversion to AIDS and Death in Europe.20
Demographic and clinical characteristics of PLHIV at entry to careWe used monthly rates of HIV testing in the model to match the age at entry to care observed in the INI/Fiocruz cohort for each group under consideration. The mean age at entry to care for the entire cohort is 34.5-years; MSW have the highest age, at 39.2-years, and TGW and MSM have the lowest, at 31.5-years for both groups (Table 1). We identified group specific CD4 counts at model outset, ranging from 635‒738 uL, so that the estimated CD4 counts at entry to care match INI/Fiocruz cohort data. The observed mean CD4 count at entry to care for the entire cohort is 353 uL; MSW have the lowest CD4 count, at 254 uL (Table 1).
HIV natural historyAge- and sex/gender-stratified non-AIDS mortality rates were estimated from the United Nations World Population Projections, the 2000‒2016 WHO Global Health Estimates, and published literature.6,23,24 We stratified rates of off-ART CD4 decline by CD4 count and HIV viral load. We derived these rates from two multicenter cohorts of untreated PLHIV.21,22 We used these HIV natural history parameters to estimate survival for PLHIV who are not receiving treatment.
ART initiation and retention in HIV careIn the model, once individuals are diagnosed with HIV, they can initiate Dolutegravir (DTG)-based ART as recommended in current Brazil guidelines.13 Second and subsequent lines of ART are available for those who do not suppress HIV RNA on first-line ART.13 Each regimen is characterized by a likelihood of HIV RNA suppression (efficacy) and monthly CD4 count increase (Table 1). Monthly probabilities of virologic suppression and of being lost to care were derived from INI/Fiocruz (Table 1).
Scenario analysisSince 2015, INI/Fiocruz has employed multiple strategies to recruit and engage TGW in HIV prevention and care, which have resulted in earlier HIV diagnosis, and as reported in a recent analysis, lower mortality rates compared to other population groups.25 This may not be representative of the experiences of TGW cared for in other health services in Brazil. Studies from other parts of Brazil have shown that TGW may avoid seeking services because of transphobic experiences which contribute to their increased vulnerability to HIV.26,27 Given these observations, we simulated a scenario where TGW presented to care with CD4 count as low as those observed for MSW (∼250 uL) to understand how LE outcomes from current care and guideline-concordant care would be affected.
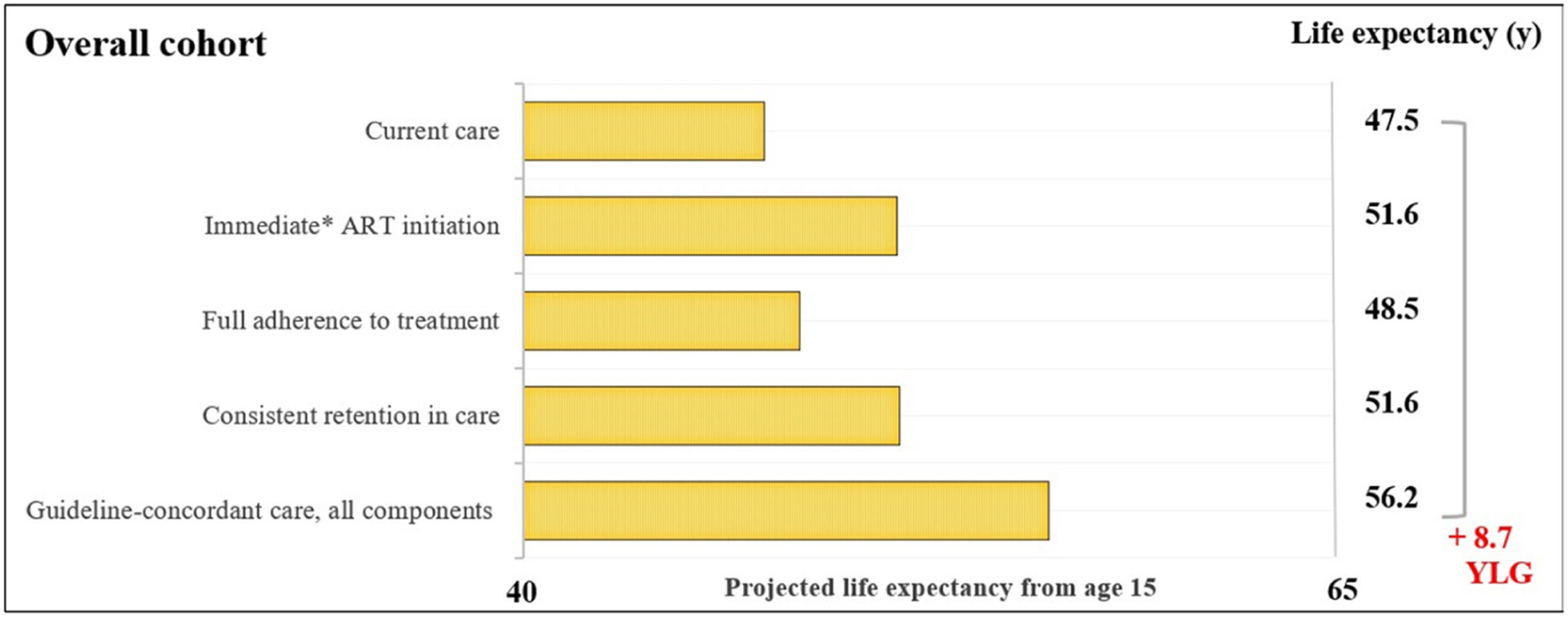
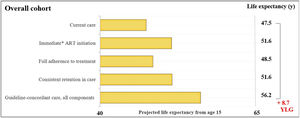
ResultsOverall cohortFor the overall cohort, the average estimated LE from age 15-years would be 47.5-years with current care and would increase to 56.2-years with guideline-concordant care (Fig. 1). Years of life lost with current care due to late initiation, suboptimal adherence, and inconsistent retention in care would be 8.7-years. We found that immediate initiation of ART upon diagnosis and consistent retention in care would result in average increases in LE of 4.1 and 4.2-years. Full adherence to treatment would result in a smaller increase of 1.1-years.
Estimated life expectancy from age 15 for people living with HIV. Estimated life expectancy (from age 15) for an overall cohort of people with HIV (PLHIV) in Brazil according to: 1) Current care, 2) Guideline-concordant care, 3) Guideline-concordant timing of ART initiation, observed adherence and retention; 4) Observed timing of ART initiation and retention with guideline-concordant adherence to treatment, and 5) Observed timing of ART initiation and adherence with guideline-concordant retention in care. Current care implies timing of treatment initiation, adherence to treatment, and retention in care as currently observed for these population groups in Brazil. Brazilian guidelines for care and treatment of PLHIV recommend initiating treatment as soon as diagnosis is made. As a conservative assumption, we assumed that the average time from HIV diagnosis to treatment initiation was 5.5-months. Abbreviations: YLG, Years of Life Gained; y, years.
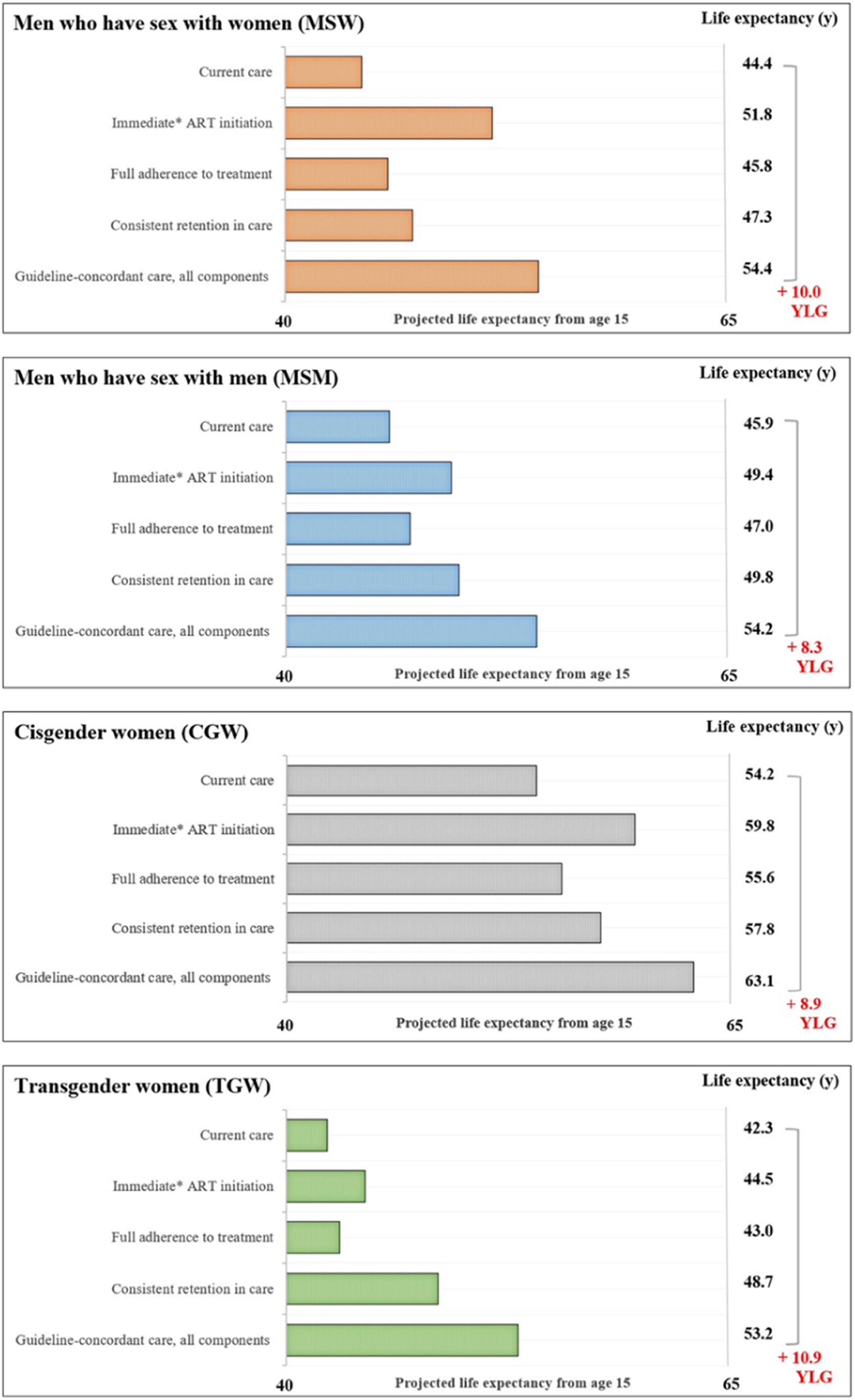
The average estimated LE from age 15 with current care would be 45.9-years for MSM, 44.4-years for MSW, 54.2-years for CGW, and 42.3-years for TGW (Fig. 2). LE estimates from age 15-years for the guideline-concordant care scenario would be uniformly higher: 54.2-years for MSM, 54.4-years for MSW, 63.1-years for CGW, and 53.2-years for TGW (Fig. 2). With guideline-concordant care, TGW would gain 10.9-years, MSW 10.0-years, CGW 8.9-years, and MSM 8.4-years, compared to current care.
Estimated life expectancy from age 15 for population groups defined by gender and sexual orientation. Estimated life expectancy (from age 15) for people with HIV stratified in four gender and sexual orientation stratified population groups: cisgender Men who have Sex with Men (MSM), cisgender Men who have Sex with Women (MSW), Cisgender Women (CGW), and Transgender Women (TGW) according to: 1) Current care, 2) Guideline-concordant care, 3) Guideline-concordant timing of ART initiation, observed adherence and retention; 4) Observed timing of ART initiation and retention with guideline-concordant adherence to treatment, and 5) Observed timing of ART initiation and adherence with guideline-concordant retention in care. Current care implies timing of treatment initiation, adherence to treatment, and retention in care as currently observed for these population groups in Brazil. As a conservative assumption, we assumed that the average time from HIV diagnosis to treatment initiation was 5.5 months. Abbreviations: YLG, Years of Life Gained; y, Years.
Assessing the impact of immediate ART initiation alone, which is defined as 5.5 months on average from HIV diagnosis to treatment initiation, MSM would gain 3.5-years of life compared to current care. MSW and CGW would gain 7.4 and 5.6 years, and TGW would gain 2.2-years. Guideline-concordant ART initiation would result in the largest improvements in LE for MSW and CGW, compared to consistent retention in care and full adherence to treatment.
Full adherence to treatment assumes 95% adherence to treatment, and would result in the smallest life years gained, compared to the other components of care and across all population groups. For this care component, there would be 1.2, 1.5, 1.4, and 0.7 life years gained for the MSM, MSW, CGW, and TGW.
Consistent retention in care assumes that no one is lost to follow up after entering care. For this care component, MSM would gain 3.9-years, MSW 2.9-years, and CGW 3.6-years. TGW would experience the largest gains from consistent retention in care, compared to improved ART initiation and full adherence to treatment, with an increase of 6.4 life years.
Scenario analysis ‒ transgender womenIf TGW presented to care with CD4 counts as low as those observed for MSW (∼250 uL), the estimated LE from age 15 with current care would decrease by 4.2-years, from 42.3-years to 38.1, and the potential LE increase could reach 15.1-years with guideline-concordant care. In this scenario, TGW would still see the largest increase in LE from consistent retention to care, compared to improved ART initiation and full adherence to treatment.
DiscussionIn this analysis, we estimated LE across different gender and sexual orientation population groups of PLHIV in Brazil, and the additional life-years that could be gained with guideline-concordant HIV care, as endorsed in Brazilian13 and international guidelines.28 We also examined the impact of individual components of guideline-concordant care, including timing of ART initiation, adherence to treatment, and retention in care. LE estimates for the guideline-concordant care scenario would be uniformly greater than LE in current care, with TGW experiencing the greatest potential increase in LE. Across all modeled population groups, we found that the largest increases in LE could be achieved with interventions aimed at earlier ART initiation and improved retention in care.
Care is needed when comparing LE estimates. Generally, LE is calculated using national mortality data, and it is often expressed as the average number of years a person can expect to live from birth. However, LE can also be calculated from different ages and, as such, it gives the average number of years a person who has already reached the specific age is expected to live. We estimated LE from age 15-years for all cohorts to fully capture HIV incidence among younger individuals and to ease comparison across the population groups of interest, and therefore projected LE for people who have already overcome the mortality risks associated with infancy and childhood. For PLHIV who acquire infection during their adult life, LE from a post-childhood age likely provides a more accurate estimate of how long someone who has already survived to that age can expect to live.
Our results corroborate current literature for LE estimates among PLHIV in Brazil and Latin America.4,29 Results based on the Caribbean, Central and South America network for HIV epidemiology (CCASAnet) data, which includes over 30,000 adults from multiple HIV clinical cohorts, estimated LE for PLHIV at 69.5-years.4 This is somewhat higher than our estimate of 62.5-years, which may be explained by the different age from which calculation was made (20-years compared to 15-years in the present study). When comparing the LE of PLHIV to that of the underlying Brazilian population, we note that PLHIV still have a much lower LE than the average, as LE from birth for the Brazilian population was estimated at 76.6-years in 2019.29 This difference may be due to the social vulnerabilities that affect PLHIV, such as lower socioeconomic status, in addition to the missed opportunities related to HIV care.
Our results show that if guideline-concordant care was achieved, LE of PLHIV in Brazil could be increased by 8.7-years, reaching 71.2-years. PLHIV are still initiating ART with CD4 counts of 350 uL on average, with our results showing that, overall, immediate ART initiation could lead to the greatest increase in LE. In terms of hospitalizations, guideline-concordant care decreased severe OIs by about three-quarters compared with actual care, suggesting that this is one of the major mechanisms by which guideline-concordant care decreased mortality. Relatedly, another analysis based on the CCASAnet collaboration estimated that 86% and 71% of all deaths in the first and fifth years following enrollment in care could have been prevented had individuals enrolled in care and initiated ART before progression to advanced HIV disease (CD4 < 200 uL).30 Unfortunately, ∼40% of PLHIV initiating ART still present with advanced disease in Brazil10 which leads to increased morbidity from opportunistic infections and contributes to high mortality during the first year of ART initiation.31 Taken together, these findings highlight the need to address barriers that hinder early diagnosis and immediate ART initiation.
When comparing current care received across the population groups, we find that TGW would have the lowest LE. Though there is limited prior research for transgender people, recent studies from England, the Netherlands, and the United States have shown that transgender people experience increased mortality compared to cisgender people.6,32 In Brazil, transphobia and discrimination can lead to unstable housing and migration, resulting in limited formal education and engagement in sex work.33-35 Additionally, TGW report avoiding health care services because of past transphobia.27,36 Among TGW living with HIV, studies have shown that this population has lower linkage to care, lower use of ART, and poorer adherence to clinical visits.37,38 For TGW, barriers to accessing care include transphobia, stigma, socioeconomic instability, and lack of awareness of available services.26 Importantly, recent experience at INI/Fiocruz has shown that providing a welcoming, gender-affirming environment with gender-neutral toilets and trans-affirming posters, for example, can improve outcomes for TGW.25
Our results highlight that LE could be higher for all population groups through improvements in HIV care, with differential impact of the components of guideline-concordant care on LE by population group. We find that MSW would benefit most from earlier ART initiation. These results are corroborated by multiple analyses, from Brazil other low and middle-income settings, which have shown that men are more likely to have severe immunodeficiency at the start of ART, as well as having other AIDS and non-AIDS-related infections.24,39 In Brazil, where access to ART is guaranteed through the public health system, late initiation of ART among men likely results from late diagnosis. MSW have much lower uptake of HIV testing: the odds of testing in the prior 12-months are 4-times higher among MSM compared to MSW.40 HIV-related stigma stands out as a major barrier to HIV testing41 which could potentially be addressed through the endorsement of universal (instead of “risk-based”) testing and HIV self-testing.
For CGW, we find that two components led to important gains in LE: immediate ART initiation and consistent retention in care. To contextualize these results within Brazil's public health system, a 2011‒2012 national study of prenatal care in Brazil that included 23,894 women showed that 99% had at least one prenatal consultation and 96% received their prenatal card, on which 82% had written results of their first HIV test.42 Moreover, among pregnant women living with HIV, 75% and 81% received ART during pregnancy and childbirth.43 As such, it is likely that most CGW in Brazil are diagnosed and initiate ART early in their adult life (adolescent pregnancy is common).44 However, due to multiple experienced vulnerabilities, these women are more likely to stop treatment and be lost to care.44 Two large studies from Brazil corroborate this hypothesis by showing that women, compared to men, had lower odds of ART use and of retention in care.7,8 As our health service at INI/Fiocruz is focused on infectious diseases, while another Fiocruz campus, Instituto Fernandes Figueira (IFF/Fiocruz), provides maternal and childcare, our data likely reflects CGW's retention in care.
For TGW, we find that improvements in retention in care led to the largest gains in LE. Corroborating these findings, studies from Brazil have shown that TGW have lower retention in both HIV care and HIV prevention services45 compared to other groups. Moreover, a recent analysis evaluating continuum of care outcomes before and during the COVID-19 pandemic showed that COVID-19 disrupted care access disproportionately for TGW.46 HIV services for TGW in Brazil should emphasize gender-affirmative care, promote autonomy, and include population-specific peer programming.47 INI/Fiocruz has actively addressed barriers to engagement in care among TGW by enabling the establishment of Transcendendo, the first trans-specific cohort in a low- or middle-income country, and facilitating the inclusion of TGW in research studies.33,35 A consequence of these strategies is that relative to the other population groups, TGW are younger and healthier at entry to care, which is likely not the case in most other health services in Brazil. Using a scenario analysis in which TGW presented to care later, we found that interventions for consistent retention in care would still result in the largest increase in LE for this population group, compared to immediate ART initiation and full adherence to treatment.
This study has several limitations. First, we used a single source to inform model parameters that describe the characteristics of the study population. Other studies from Brazil could have informed some parameters with nationally representative sources. However, most national studies do not disaggregate data according to gender and sexual orientation and, therefore, cannot inform the different experiences for the studied groups. The cohort at INI/Fiocruz has detailed gender and sexual orientation information, which we have used to understand how the experiences of these population groups differ.24,25 Another limitation of the present study is the lack of regional variation in mortality and other key model parameters that might more accurately reflect Brazil's heterogeneity; thus, our analysis cannot address variation in survival that may result from underlying LE differences between regions. We also did not specifically examine the benefits of tuberculosis prophylaxis as distinct from ART. Tuberculosis is a leading cause of death among PLHIV in Brazil48 and future studies could examine the potential increase in life-expectancy among PLHIV from tuberculosis prophylaxis. Additionally, we chose not to model people without HIV and because of this, we do not account for disparities in HIV incidence or the fact that some population groups are more susceptible to HIV infection due to structural inequities. Finally, we did not include transmissions in this analysis, though the value of guideline-concordant care in preventing further transmission would add to the individual benefits we describe.
ConclusionsIn Brazil, HIV disproportionately burdens vulnerable populations. Although HIV testing, care and treatment are provided free-of-charge, actual care does not always meet recommended guidelines and varies greatly by gender and sexual orientation; this results in LE disparities between different gender and sexual orientation groups. Moreover, we noted that different components of HIV care could benefit specific population groups differently. These results suggest that interventions should be tailored to population groups and their specific HIV care needs since ‘one size does not fit all.’ In settings such as INI/Fiocruz, MSW and CGW would benefit most from earlier diagnosis and improved linkage to care, whereas TGW and MSM would benefit most from sustained engagement in care. Assessment of the HIV care continuum for specific populations should inform care priorities.
Ethics approvalThis study was approved by the Institutional Review Board at Instituto Nacional de Infectologia Evandro Chagas (INI) of Fundação Oswaldo Cruz (FIOCRUZ), 38714114.3.0000.5262.
Consent to participateNo patient-level data were included in this modeling study so no consent was required.
Availability of data and materialAvailable from corresponding authors upon reasonable request.
Code availability (software application or custom code)Details of CEPAC models are available at www.massgeneral.org/medicine/mpec/research/cpac-model.
Authors’ contributionsPML, HS, JAS, BG, VGV, KAF, and EL conceived and designed the study. PML, HS, and EL performed the statistical analyses. PML and HS performed model runs and created the tables and figures. PML, EL, and KAF revised results. PML, HS, JAS, KAF, and EL analyzed and interpreted the findings. PML, HS, KAF, and EL drafted the article. All authors critically revised the article for important intellectual content. All authors read and approved the final manuscript.
FundingThis work was supported by the National Institute of Allergy and Infectious Diseases (R37 AI058736), National Institute of Mental Health (R01 MH105203), and National Institute on Drug Abuse (R37 DA015612). PML and BG acknowledge funding from the National Council of Technological and Scientific Development (CNPq) and the Research Funding Agency of the State of Rio de Janeiro (FAPERJ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health or other funders.