Perinatally HIV-infected children are surviving into adulthood, and getting pregnant. There is a scarcity of information on health and pregnancy outcomes in these women.
AimTo evaluate characteristics related to HIV disease and pregnancy outcomes in perinatally infected women, and to compare these women with a group of youth with behaviorally acquired HIV-infection, at a reference hospital in Rio de Janeiro, Brazil.
MethodsA cohort study. Epidemiological, clinical, and laboratory data were compared between perinatally (PHIV) and behaviorally HIV-infected (BHIV) pregnant youth with the primary aim to study pregnancy outcomes in the PHIV group and compare with outcomes to BHIV group.
ResultsThirty-two pregnancies occurred in PHIV group, and 595 in BHIV group. A total of seven (22%) PHIV women and 64 (11%) BHIV women had a premature delivery (p=0.04), however, when adjusting for younger age at pregnancy, and antiretroviral therapy initiation in 1st trimester of pregnancy (OR=18.66, 95%CI=5.52–63.14), the difference was no longer significant. No cases of mother-to-child HIV transmission (MTCT) were observed in the PHIV group while there was a 2% MTCT rate in BHIV group.
ConclusionPregnancy among PHIV was as safe as among BHIV. The differences between those groups were probably related to treatment and prolonged care in the first group.
Mother-to-child transmission of HIV (MTCT) is the most common route of HIV infection in children. However, with adequate antiretroviral treatment (ART), antenatal and delivery care, the risk of HIV MTCT has reduced from a previous reported rate of 15–40%1–4 to less than 1%.5 It has been shown that even in cases where the HIV infected mother does not receive ART the risk of HIV MTCT can be significantly reduced with effective perinatal and neonatal care.
Risk factors for HIV MTCT include maternal immunosuppression, high viral load at labor, inadequate use of antiretroviral therapy, premature delivery, chorioamnionitis, other sexually transmitted infections, time of rupture of membranes, low birth weight, breast-feeding, and birth delivery mode.4,6,7
In 2010, in Brazil, 2 per 1000 live births were HIV-exposed, i.e., HIV infection was detected in the mother during antenatal care (ANC) or later. In 12 years there has been 40.7% reduction in incidence of HIV in children under the age of five years in this country.8
While pregnancy in HIV-infected women in general has been extensively studied, there are few studies conducted on perinatally infected pregnant women. This group of younger women who were infected by their mothers at birth is surviving9–11 and facing the desire to form a family. From studies published so far, it seems as though the MTCT rate was low, between 0 and 7.7%12–19 in the group of perinatally infected pregnant women, although viral suppression20,21 was not obtained in many cases. There are reports of higher proportion of babies born with low birth weight in this group,22 higher rates of premature delivery,15,16 but these results are contradicted by others.13,17
Another important issue observed on these reports are a high incidence of elective abortion reported in 14/33 cases13 and 14/34 cases.16 Therefore, more studies are necessary before any definitive conclusions can be drawn about this patient group.
This study aimed to compare demographic, clinical, and pregnancy outcome characteristics of young pregnant women who acquired HIV perinatally or behaviorally.
MethodsThis study is a cohort study of young HIV-infected pregnant women who had been referred to Instituto de Puericultura e Pediatria Martagão Gesteira (IPPMG) Antenatal Clinic from 1997 to 2013. Inclusion criteria for this study were: being HIV infected; age below 25 years old; being pregnant; and followed-up for at least two ANC visits at the IPPMG Clinic. There were no cases of repeat pregnancies in the cohort.
By using a validated case report form patient data were prospectively obtained from HIV-infected pregnant women who presented at the ANC facility. The health care professional responsible for the care of the patient filled in the forms based on patient's responses, laboratory results and medical records. Information from these report forms was thereafter manually transferred into different variables of interest.
The primary aim of the study was to compare differences in pregnancy outcomes between the groups of perinatally and behaviorally HIV-infected pregnant women. Perinatally infected were those with a known HIV positive mother diagnosed during the first 18 months after birth who was referred from a pediatric/adolescent clinic with history of perinatal infection and without history of any other source of infection. Behaviorally infected were those diagnosed as HIV infected with a previous history of sexual debut or intravenous drug use. Combined antiretroviral treatment (cART) was defined as a regimen combining at least three different antiretroviral drugs from at least two different antiretroviral classes.
The two groups were compared regarding household income, time of initiation of cART, CD4 cell count and HIV viral load at admission to ANC and close to delivery, initiation of cART before pregnancy, initiation of cART in 1st trimester of pregnancy.
Route of HIV transmission was set as the main exposure variable. The outcome variables studied were premature delivery (prematurity defined as gestational age at birth <37 weeks assessed by Capurro methodology), opportunistic infections during pregnancy, STI during pregnancy (including history of HPV/cervical intraepithelial neoplasia, syphilis), induced abortion, spontaneous abortion, birth weight (z-scored for age and sex, based on WHO, 2006 charts), elective cesarean section/vaginal delivery, premature amniorhexis, stillbirth, CD4 cell count and HIV viral load close to delivery, cesarean-section wound infection, and HIV-infection of the babies. Perinatally HIV-infected (PHIV) and behaviorally HIV-infected women (BHIV) were compared using the Student's t-test for continuous variables and Chi-square or Fisher exact tests for categorical variables.
To evaluate the change in CD4 cell count and viral load from admission to ANC through delivery in the two HIV-infected groups, paired Student's t-test was used. Variables with a p-value <0.20 in univariate analysis were then included in the multivariate analysis. A multivariate binary logistic regression analysis was performed. Differences with p-value <0.05 were regarded statistically significant.
This data was processed and analyzed using SPSS statistics version 17.0.
Ethical permission to use patient data in this study was approved by IPPMG Ethical Committee.
ResultsA total of 630 pregnancies in HIV-infected women under the age of 25 were included in the study. Thirty-two occurred in the PHIV and 595 in the BHIV groups. The overall median age was 21 years (inter-quartile range 17–23). In three cases, data about routes of HIV infection were missing, and the patients were excluded from the analysis. There were no cases of repeat pregnancies in the cohort, but some spontaneous and induced abortions were observed (Table 1).
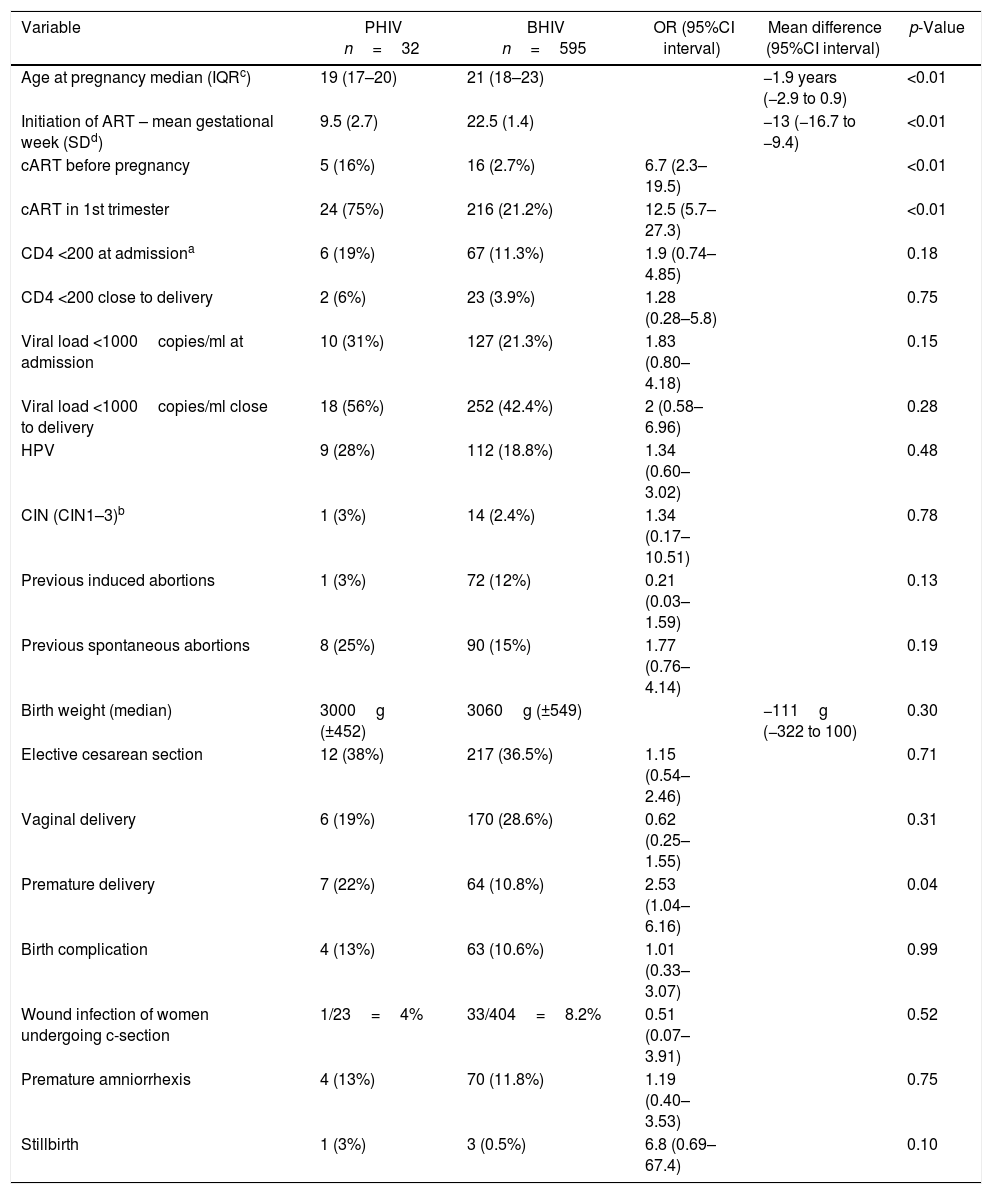
Univariate analysis – differences between perinatally (PHIV) and behaviorally (BHIV) HIV infected pregnant women.
| Variable | PHIV n=32 | BHIV n=595 | OR (95%CI interval) | Mean difference (95%CI interval) | p-Value |
|---|---|---|---|---|---|
| Age at pregnancy median (IQRc) | 19 (17–20) | 21 (18–23) | −1.9 years (−2.9 to 0.9) | <0.01 | |
| Initiation of ART – mean gestational week (SDd) | 9.5 (2.7) | 22.5 (1.4) | −13 (−16.7 to −9.4) | <0.01 | |
| cART before pregnancy | 5 (16%) | 16 (2.7%) | 6.7 (2.3–19.5) | <0.01 | |
| cART in 1st trimester | 24 (75%) | 216 (21.2%) | 12.5 (5.7–27.3) | <0.01 | |
| CD4 <200 at admissiona | 6 (19%) | 67 (11.3%) | 1.9 (0.74–4.85) | 0.18 | |
| CD4 <200 close to delivery | 2 (6%) | 23 (3.9%) | 1.28 (0.28–5.8) | 0.75 | |
| Viral load <1000copies/ml at admission | 10 (31%) | 127 (21.3%) | 1.83 (0.80–4.18) | 0.15 | |
| Viral load <1000copies/ml close to delivery | 18 (56%) | 252 (42.4%) | 2 (0.58–6.96) | 0.28 | |
| HPV | 9 (28%) | 112 (18.8%) | 1.34 (0.60–3.02) | 0.48 | |
| CIN (CIN1–3)b | 1 (3%) | 14 (2.4%) | 1.34 (0.17–10.51) | 0.78 | |
| Previous induced abortions | 1 (3%) | 72 (12%) | 0.21 (0.03–1.59) | 0.13 | |
| Previous spontaneous abortions | 8 (25%) | 90 (15%) | 1.77 (0.76–4.14) | 0.19 | |
| Birth weight (median) | 3000g (±452) | 3060g (±549) | −111g (−322 to 100) | 0.30 | |
| Elective cesarean section | 12 (38%) | 217 (36.5%) | 1.15 (0.54–2.46) | 0.71 | |
| Vaginal delivery | 6 (19%) | 170 (28.6%) | 0.62 (0.25–1.55) | 0.31 | |
| Premature delivery | 7 (22%) | 64 (10.8%) | 2.53 (1.04–6.16) | 0.04 | |
| Birth complication | 4 (13%) | 63 (10.6%) | 1.01 (0.33–3.07) | 0.99 | |
| Wound infection of women undergoing c-section | 1/23=4% | 33/404=8.2% | 0.51 (0.07–3.91) | 0.52 | |
| Premature amniorrhexis | 4 (13%) | 70 (11.8%) | 1.19 (0.40–3.53) | 0.75 | |
| Stillbirth | 1 (3%) | 3 (0.5%) | 6.8 (0.69–67.4) | 0.10 |
At pregnancy, PHIV women were younger, median age 19 years, and 22 in BHIV women (Table 1). This age difference remained significant even when comparing only primigest women (p=0.01).
The mean household income was 1.83 minimum salaries in PHIV group and 2.20 in BHIV group. The difference in income between the two groups was thus small and not statistically significant (p=0.48).
Pregnant PHIV had a tendency to have lower initial CD4 cell count and viral load at admission, although these differences did not reach significance (Table 1). A total of 31% and 21% had a viral load below 1000copies/ml at admission, in the PHIV and BHIV groups, respectively (Table 1).
There was a significant improvement in CD4 cell counts and viral load between admission to ANC and delivery in both groups: in PHIV group, CD4 cell count increased 125 on average (p<0.01), and viral load decreased 4.2 log (p<0.01) on average; in the BHIV group, the mean increment of CD4 cell count was 98 (p<0.01), and the mean viral load decrement was 4.5 log (p<0.01). Nonetheless, only 56% of the PHIV group had a CD4 cell count mean increase of 125 (p<0.01) and a mean viral load decrease of 4.2 log (p<0.01). In the BHIV group, 42.4% reached a viral load below 1000copies/ml close to delivery.
PHIV women started antiretroviral therapy earlier than BHIV women. In the PHIV group, 25 women were on cART during pregnancy (of which 24/25 women were on cART from 1st trimester), six women were dual therapy and one woman on monotherapy. Among PHIV women who received cART, 23 were on PI-based cART and two women were on NNRTI based cART. In the BHIV group, 300 women were on cART during pregnancy (of which 216 were on cART from 1st trimester), 248 women were on PI-based cART, 50 women on NNRTI-based cART and two used both PI- and NNRTI-based cART.
A history of opportunistic infections during pregnancy was reported in 3% of women in both groups (Table 1). Previous induced abortion tended to be more common in BHIV group, while previous spontaneous abortion was slightly more common in PHIV group.
Although this study evaluated women younger than 25 years-old, cervical intraepithelial neoplasia (CIN) grades 1–3 were found in 3% and 2.4% of PHIV and BHIV women, respectively.
PHIV women tended to give birth at a mean of 1.1 weeks earlier (p=0.20). Premature delivery was more common among PHIV women (22%) compared to BHIV women (10.8%) (p=0.04) (Table 1). However, when adjusting for cART initiation in the first trimester and age at pregnancy, prematurity was not significantly different between the two groups. There was no significant difference in birth weight of those born to women of the two groups (Table 2). The rates of elective cesarean section were almost the same in both groups while vaginal delivery tended to be more common in the BHIV women. This difference was not significant (Table 1).
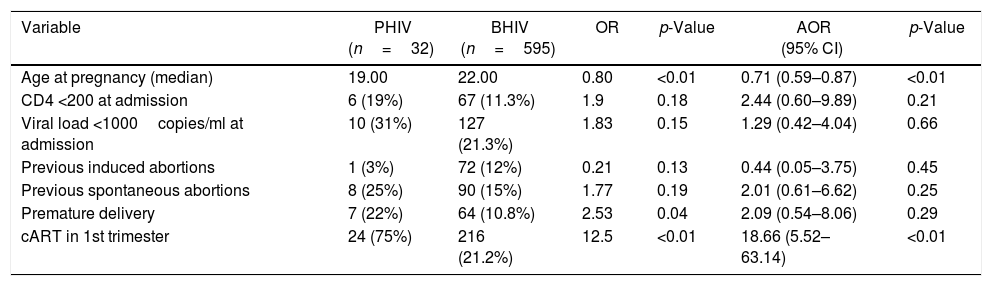
Multivariate analysis – differences between perinatally (PHIV) and behaviorally (BHIV) HIV infected pregnant women.
| Variable | PHIV (n=32) | BHIV (n=595) | OR | p-Value | AOR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Age at pregnancy (median) | 19.00 | 22.00 | 0.80 | <0.01 | 0.71 (0.59–0.87) | <0.01 |
| CD4 <200 at admission | 6 (19%) | 67 (11.3%) | 1.9 | 0.18 | 2.44 (0.60–9.89) | 0.21 |
| Viral load <1000copies/ml at admission | 10 (31%) | 127 (21.3%) | 1.83 | 0.15 | 1.29 (0.42–4.04) | 0.66 |
| Previous induced abortions | 1 (3%) | 72 (12%) | 0.21 | 0.13 | 0.44 (0.05–3.75) | 0.45 |
| Previous spontaneous abortions | 8 (25%) | 90 (15%) | 1.77 | 0.19 | 2.01 (0.61–6.62) | 0.25 |
| Premature delivery | 7 (22%) | 64 (10.8%) | 2.53 | 0.04 | 2.09 (0.54–8.06) | 0.29 |
| cART in 1st trimester | 24 (75%) | 216 (21.2%) | 12.5 | <0.01 | 18.66 (5.52–63.14) | <0.01 |
The rate of pregnancy complications and concomitant diseases were similar in the two groups. There were 36 cases of surgical wound infection, six cases of syphilis, one case of rash of unknown cause, one case of leprosy, three cases of pregnancy related hypertension, four cases of urinary tract infections, four cases of anemia/bleeding, and one case of headache after epidural anesthesia.
The most common complication was surgical site infection after cesarean section (4.3% in PHIV and 8.2% in BHIV).
No infants born to PHIV mothers were infected with HIV (1/32 infant lost to follow up), whereas 2% of babies born to mothers in BHIV group were found to be HIV-infected (29/595 lost to follow up).
DiscussionIn this study, characteristics of pregnant adolescents and young adult women with perinatally acquired HIV infection were compared to a cohort of women with behaviorally acquired HIV-infection. The findings of this study indicate that maternal health and pregnancy outcomes were similar between the two groups, although some differences seem to exist.
Initiation of cART in 1st trimester more common in PHIV group can probably be attributed to the fact that the women in this group are HIV infected from birth and therefore have been given HIV health care since early childhood. Premature delivery was found to be more common among women with perinatal HIV infection, although without statistically significant difference after adjusting for initiation of cART in first trimester and age. HIV MTCT transmission occurred in 2% of cases in BHIV group whereas no cases of MTCT HIV transmission occurred in the PHIV group.
The group of perinatally HIV infected women was significantly younger at pregnancy. These findings are in line with other studies in which vertically and behaviorally HIV-infected women have been compared.16,23 In this study the rates of STIs were high in both groups (mainly HPV and syphilis), which could also be a reflection of a high rate of sexual risk behavior, possible related to neurodevelopmental and cognitive problems leading to hyperactivity and/or impulsivity.24,25 This is however in contrast to studies that showed lower rate of sexual risk behavior in adolescents with perinatal HIV transmission than in uninfected youth.26 It is also important not to draw any conclusions toward characterizing these teenagers and young adults as problematic and incapable of taking responsibility for their disease and reproductive health.
Though not significantly different, there was a trend for PHIV to be more likely to achieve viral suppression at delivery than BHIV women. This may be attributed to the fact that they were more likely to start cART prior to pregnancy or during 1st trimester. Antiretroviral treatment during ANC was effective with significant decreases in viral load and increases in CD4 cell counts in both cohorts. However, only 42–56% of the women were able to reach viral load <1000copies/ml before delivery, rates lower than in other studies.5,21 The reason for this result should be multifactorial. Since we included women followed since 1997, less potent ARVs were used, adherence to ARVs tend to be lower among adolescents and young adults, and it is likely that part of PHIV women were already ARV experienced, in contrast to the majority of BHIV women who were ARV naive.26
It may seem surprising that only 16% of PHIV women were on cART prior to pregnancy. A possible explanation for this finding may be that those who survived into adulthood with good health and became pregnant were long term non-progressors. Furthermore, this was at a time when the benefit of early antiretroviral treatment had not been proven which also might explain the low rate of cART in the PHIV group.
In spite of many women presenting with high viral loads and CD4 cell counts <200cells/mm3, a history of opportunistic infection was uncommon in both groups, demonstrating that HIV-related health status was generally good.
The incidence of cervical intraepithelial neoplasia (CIN) in the pregnant women was around 3% in both groups. This is an incidence rate higher than that reported in pregnancy in general where a CIN incidence between 0.06 and 1% was reported.27,28
The prevalence of HPV was 28% in PHIV group and 19% in BHIV group. This is within the wide range of HPV prevalence previously reported from Brazil. Since no analysis of which type of HPV serotype was available we do not know if the high rate of CIN could be caused by a high prevalence of cancer-associated HPV-strains or whether it is a reflection of a higher susceptibility to cervical neoplasia because of other reasons.29,30
No association between previous induced abortions and perinatal acquisition of HIV was found, in contrast to other studies that found a high incidence of elective termination of pregnancy in women with this route of HIV infection.12,15 In this study, only one woman (3%) in PHIV group had a history of induced abortion. Studies have shown a higher frequency of pregnancy desire in HIV-infected women who are aware of the low risk of HIV MTCT31 and who have stronger belief in the efficacy of measures to reduce HIV MTCT.32 Women who have lived with the HIV-disease since childhood are more likely to be well educated and informed about their disease and therefore aware of the risks and possibilities for pregnancy while infected with HIV. It is also important to note that abortion is illegal in Brazil, and a low rate of abortion could therefore be explained by lack of access to safe termination of pregnancy, as well as underreporting.
A history of spontaneous abortions on the other hand tended to be more common in the PHIV group. Spontaneous abortion has been previously reported in some studies to be more common in HIV-infected women16,33 whereas other studies found no such difference between infected and uninfected women.34
Premature delivery seemed to be significantly more likely to occur in PHIV women, but this association lost significance after adjustment for early start of cART and age. The explanation for this higher frequency of prematurity might be that PHIV mothers were more likely to initiate cART before pregnancy/in 1st trimester of pregnancy, which has been reported to increase the risk for premature delivery.35,36 Nonetheless, this is something that future research will have to investigate. Rough endpoints like extremely low birth weight and stillbirth were rare in both groups.
The rate of MTCT of HIV was low in both cohorts. This was an encouraging result, in line with contemporary studies,37 where the best antiretroviral treatment and antenatal care has been given. The relatively small cohort of perinatally infected women might also give a skewed result of the actual risk of HIV transmission in this group. The results from this study does however contribute to the encouraging picture of pregnancy in women who acquired HIV at birth, confirming results showing that third-generation transmission of HIV is uncommon.
A weakness of this study is the potential of heterogeneity of the control group of behaviorally infected women, considering that it includes women who acquired HIV through different ways, as well as the number lost to follow-up. However, the absolute majority in the group acquired HIV through sexual exposure. Also the study does not account for possible confounding factors such as previous history of preterm birth, race, and substance abuse during pregnancy that could have affected outcome of preterm birth.
In conclusion, this is the largest study that has been conducted on pregnancy in women with perinatal HIV-infection in Brazil. Considering that over 14,000 cases of AIDS have been registered among children under the age of five years since the emergence of the AIDS epidemic in the beginning of the 1980s, one can expect an increasing number of pregnancies in this group. It is therefore of importance to study the health and pregnancy outcomes and try to identify possible challenges that are unique to this group compared to women who acquired HIV through different modes.
The concerns about the unique challenges that confront women with perinatal HIV-infection are relevant and important to address in further future research. As described earlier, these include neurocognitive problems, drug resistance, and social problems.36,37 However, the emerging evidence from this study, as well as from previous studies on pregnancies in perinatally infected youth give us reason to believe that most of these women are in fairly good health and are highly capable of managing their disease and treatment, and that they will give birth to healthy babies, avoiding the emergence of large group of third generation HIV-infected infants.
Conflicts of interestThe authors declare no conflicts of interest.
PL received a grant from Sten A Olsson foundation for research and culture CBH received a grant from CNPq – Brazil – PQ2.