Three isolates of Streptococcus agalactiae, recovered from residents of the metropolitan area of Rio de Janeiro with significant bacteriuria, were found to be resistant to levofloxacin. Determination of the minimal inhibitory concentration (MIC) confirmed one isolate as intermediate and two as resistant to levofloxacin. No reduction in levofloxacin MIC was observed with reserpine, indicating that resistance was not caused by an efflux mechanism. Typical point mutations were observed in the quinolone resistance determinant region of gyrA and parC. Other point mutations in parC generated novel altered codons: Ser80→Pro in the intermediate resistance isolate, and Gly128→Asp in a resistant isolate. Through molecular modeling, it was possible to observe that these novel substitutions might not play a role in resistance, since these amino acids were not involved in the antibiotic binding site. Pulsed field gel electrophoresis profiles revealed a non-clonal trend among these isolates. This is the first report of genetic characterization of levofloxacin-resistant S. agalactiae strains in Brazil.
Streptococcus agalactiae (group B Streptococcus, GBS) is one of the most important agents of infectious diseases in newborns, causing life-threatening diseases such as meningitis and septicemia.1 GBS is also responsible for infections in non-pregnant individuals, especially elderly and those with certain underlying conditions.2
Penicillin remains the first choice to treat GBS infections, although strains with reduced susceptibility to this antimicrobial agent have been recently described.3 Conversely, GBS resistance to alternative therapy, such as macrolides and lincosamides, has emerged in the last decades. Fluoroquinolone resistance in Streptococcus agalactiae was first described in Japan in 2003,4 and after that, resistant isolates were also recovered in other countries, although as rare events.5–8 The major mechanism of fluoroquinolone resistance among GBS is related to point mutations in the quinolone resistance determinant region (QRDR) of gyrA, leading to amino acid substitutions (Ser-81→Leu; Glu-85→Ala or Lys) and parC (Ser-79→ Phe, Ala or Tyr).4,5 Such substitutions, in most cases, decrease the affinity of the antibiotic with the enzyme, because the change in amino acid hampers the interactions of the antibiotic in the binding site. Active efflux has been addressed as a fluoroquinolone resistance mechanism in Streptococcus pneumoniae,9 but has never been described in S. agalactiae.5,6
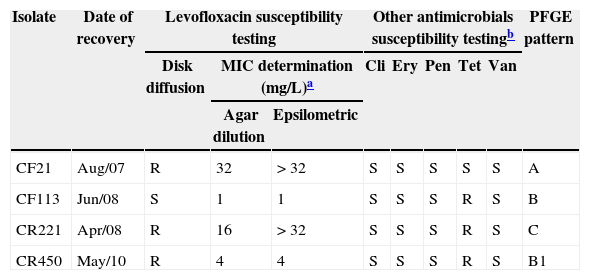
During a recent antimicrobial susceptibility survey conducted in Rio de Janeiro by this group, Nakamura et al.10 found one clinical GBS isolate resistant to fluoroquinolones, but no genetic characterization was performed in this previous study. Here, the phenotypic and genetic characteristics of three levofloxacin non-susceptible GBS isolates circulating in this geographical area are described.GBS isolates (n=500), identified by phenotypic tests (beta-hemolytic Gram-positive cocci, catalase negative, CAMP factor positive, hippurate hydrolysis test positive, streptococcal serological test positive to group B)11 were recovered from several specimens for routine diagnosis in two clinical laboratories (Instituto Fernandes Figueira and Fleury Group, Rio de Janeiro) from 2007 to 2010. All isolates were submitted to susceptibility testing to recommended antimicrobial agents by disk diffusion method.12 Three epidemiologically unrelated isolates of GBS, susceptible to clindamycin, erythromycin, penicillin, and vancomycin, were found to be resistant to levofloxacin (Table 1). These isolates were recovered from the urine of patients with significant bacteriuria (> 105 CFU/mL). One isolate (CF21) was recovered in 2007 from a hospitalized 15-year-old female previously submitted to renal surgery. Two isolates were recovered from outpatients, a 66-year-old female in 2008 (CR221), and a 56-year-old male in 2010 (CR450). This latter patient had used norfloxacin prior to the GBS positive culture. A levofloxacin susceptible isolate (CF113) was included for comparison. This isolate was recovered from a 19-year-old pregnant patient with significant bacteriuria. Determination of levofloxacin minimal inhibitory concentration (MIC) by agar dilution13 and epsilometric methods (MIC Evalutator, Oxoid – Basingstoke, UK) confirmed two isolates as resistant and one as intermediate. Such discrepancy when using different antimicrobial susceptibility test methodologies was previously reported in GBS isolates.14 No change in levofloxacin MIC values was observed when reserpine (70mg/L) was incorporated in the medium, suggesting that the active efflux pump system was not involved in fluoroquinolone resistance. Continuous GBS antimicrobial susceptibility surveys conducted in the last years allowed the detection of three (0.6%) non-susceptible levofloxacin strains, recovered from residents of the metropolitan area of Rio de Janeiro with significant bacteriuria. In other studies, levofloxacin resistance has been reported at a frequency of 1% of GBS isolates, mainly recovered from respiratory and urinary tract specimens.4–7
Antimicrobial susceptibility testing and pulsed field gel electrophoresis (PFGE) patterns of group B Streptococcus (GBS) isolates.
| Isolate | Date of recovery | Levofloxacin susceptibility testing | Other antimicrobials susceptibility testingb | PFGE pattern | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Disk diffusion | MIC determination (mg/L)a | Cli | Ery | Pen | Tet | Van | ||||
| Agar dilution | Epsilometric | |||||||||
| CF21 | Aug/07 | R | 32 | > 32 | S | S | S | S | S | A |
| CF113 | Jun/08 | S | 1 | 1 | S | S | S | R | S | B |
| CR221 | Apr/08 | R | 16 | > 32 | S | S | S | R | S | C |
| CR450 | May/10 | R | 4 | 4 | S | S | S | R | S | B1 |
Amplification and sequencing of internal regions of gyrA and parC genes were performed as described elsewhere.15 Sequences were deposited in the GenBank database and accession numbers for gyrA and parC were HQ687739 to HQ687746. Analysis of deduced amino acids sequences was performed using the software CLC Sequence Viewer (CLC BioA/S). Point mutations were detected in internal regions of both gyrA and parC genes. Resistant and intermediate isolates had Ser-81→Leu in gyrA. In parC, Ser-79→Phe was observed in both resistant isolates, whereas the intermediate isolate had another substitution (Ser-80→Pro). An additional substitution in parC (Gly-128→Asp) was observed in one resistant isolate (CR221).
In order to check whether the substitution Ser-80→Pro had an effect in MIC increase, molecular interaction between levofloxacin and topoisomerase IV was simulated. The protein topoisomerase IV complexed with levofloxacin was taken from the Protein Data Bank (PDB) under the code 3K9F with resolution of 2.90Å.16 The complex was visualized using the program Molegro Molecular Viewer 2.2.0 (Molegro ApS). It was observed that the hydroxyl group of the amino acid residue Ser79 interacted through a strong hydrogen bond with the carboxylic acid group of levofloxacin molecule at a distance of 3.18Å, while the distance of interaction between the drug and Ser80 residue was 5.80Å, what did not feature a hydrogen bond. This probably indicates that this mutation (Ser-80→Pro), which is new in GBS, is not implicated in the resistance mechanism. Also, Ser-80→Pro has been observed in levofloxacin susceptible isolates of Streptococcus pyogenes.17 Therefore, the cause of the intermediate levofloxacin isolate might be the gyrA mutation, since a MIC increase can be explained by mutation in either gyrA or parC.6 An additional substitution (Gly-128→Asp) was observed in one resistant isolate (CR221). It was observed that the residue Gly128 was located far from levofloxacin (c.a.26.5Å), and thus, there was no possible interaction between drug and amino acid.
DNA polymorphism profiles of levofloxacin susceptible and non-susceptible isolates were obtained after digestion with SmaI and separation of the fragments by pulsed field gel electrophoresis (PFGE), as previously described.18 DNA profiles were compared by visual inspection and the genetic relatedness was determined according to Tenover et al.19 Analysis of restriction profiles revealed a non clonal trend among levofloxacin non-susceptible isolates. However, related profiles (two fragment differences) were observed between the susceptible and the intermediate isolates, suggesting that despite the lack of epidemiological link among them, they may have a close genetic relation. Conversely, the patient whose intermediate resistance GBS isolate was recovered had recently used norfloxacin, which could have contributed to the selection and spread of isolates that showed an increase in levofloxacin MIC but remained closely related to the susceptible isolate. While some studies observed distinct PFGE profiles among fluoroquinolone resistant isolates,5,7 Murayama et al.15 demonstrated a high level of similarity among them. Also, Faccone et al.8 observed two clones among nine resistant isolates from Argentina. These results suggest that fluoroquinolone resistance in GBS may be related to the spread of some clones or to genetically distinct strains.
Characterization of fluoroquinolone resistant GBS circulating in Rio de Janeiro allowed the conclusion that point mutations in QRDR are the major resistance mechanism, and they may occur in a non-clonal trend. Although low levels of resistance have been observed worldwide, a clinical concern is the high frequency of the use of fluoroquinolones to treat UTIs, which may contribute to select resistant strains, especially considering GBS as a frequent uropathogen.
Conflict of interestAll authors declare to have no conflict of interest.
The authors thank Selma Romeu Souza, Group Fleury, for the donation of strains; Maria Cristina Silva Lourenço and the Plataforma Genômica – Sequenciamento de DNA - PDTIS/Fundação Oswaldo Cruz (RPT01A), Rio de Janeiro; Pró-Reitoria de Pesquisa, Pós-Graduação e Inovação, Universidade Federal Fluminense and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro/FAPERJ, grant E-26/ 170.261/2006.