Trypanosomatids are an important group of parasites that predominate in tropical and subtropical areas of the planet, which cause diseases that are classified as forgotten and neglected by the world health organization. In this group of parasites, we find Trypanosoma cruzi, Trypanosoma brucei, Trypanosoma brucei rhodesiense and Leishmania spp, for which there is no vaccine available, and its control has focused mainly on pharmacological treatment. Due to the poverty situation where these diseases are found and the biological complexity of these parasites, there are multiple variables to control, including the diversity of species, the complexity of their life cycles, drug resistance, cytotoxicity, the limited use in pregnant women, the high costs of treatment and the little-known pharmacological mechanisms of action, among others. It is therefore necessary to find new strategies and approaches for the treatment of these parasitic diseases. Among these new approaches is the rational search for new targets based on the allosteric inhibition of protein kinases, which have been little studied in trypanosomatids. Among these kinases, we find Glycogen Synthase Kinase-3 (GSK-3), a kinase of great pharmacological interest, which is under intense basic and clinical research by pharmaceutical companies for the treatment of cancer. This kinase, highly studied in the PI3K/AKT/mTOR pathway signaling in humans, has an orthologous gene in these parasites (GSK-3 s), which has proven to be essential for them in response to different challenges; Therefore, it is notable to increase research in this kinase in order to achieve a broad structural and functional characterization in the different species of trypanosomatids.
Trypanosomatids are a group of tropical parasites, belonging to the Trypanosomatidae family, which are a major problem for global public health; Among them are different species of Leishmania, belonging to the subgenera Viannia y Leishmania.1 These protozoans cause various clinical manifestations in humans and animals, such as cutaneous, mucosal and visceral leishmaniasis.1 In this group, we also find Trypanosoma cruzi, a parasite that causes Chagas disease in the Americas, a disease that manifests itself systemically and chronically.2 Among these parasites, on the African continent, human African trypanosomiasis stands out, caused by Trypanosoma brucei and Trypanosoma brucei rhodesiense, which can mainly affect the central nervous system; These pathologies together are part of the twenty forgotten and neglected tropical diseases that the World Health Organization (WHO) aims to eradicate by 2030.3 Leishmaniasis is transmitted to humans by the bite of female sandfly mosquitoes, which inoculate metacyclic promastigotes in the skin and infect mainly macrophage-like cells.4 Human infection is caused by 21 of the 30 species that infect mammals and are present in approximately 88 countries, home to 350 million people who are at constant risk of infection.5 The natural transmission of Trypanosoma cruzi in the Americas through triatomine insects causes around twelve thousand deaths a year and many babies are born infected.6 In 1980, Chagas disease was considered one of the five leading causes of morbidity and mortality in the Americas.7 These diseases, considered forgotten and neglected, have no vaccine available and their control has focused primarily on pharmacological treatment and vector control, due to their difficult social environment and biological complexity; which includes a wide variety of species, complex life cycles, drug resistance, drug toxicity, limited use in pregnant women, high costs, poor availability in endemic places, poorly understood pharmacological mechanisms of action, little investment in development of vaccines and the shortage of drugs in endemic areas to treat these diseases in the last 80 years.7
For this reason, it is necessary to find new approaches for the treatment of these pathologies. One of them is the rational search for new targets based on the allosteric inhibition of kinases, present in these parasites and little studied in these protozoans. Protein kinases are enzymes that play a crucial role in the regulation of various cellular functions, including signal transmission, cell division, cell survival, and response to growth factors.8 In the context of therapy, kinases are often studied and postulated as new therapeutic targets due to their involvement in various diseases, including cancer.9 These proteins are enzymes that phosphorylate other proteins once they recognize their substrate, generating a conformational change that translates into a cellular signal.9 There is a large number of kinases, which participate in specific metabolic pathways for survival and differentiation.10 Kinase-targeted therapy involves the development of drugs that specifically inhibit the activity of a particular kinase, which can negatively affect cell growth and survival.10 Some well-known examples of kinase-targeted therapies include tyrosine kinase inhibitors, used in cancer treatment, such as imatinib (Gleevec), which is used in chronic myeloid leukemia and other types of cancer.11
GSK-3β kinase and its relationship with the PI3K/AKT/mTOR pathwayIn humans, the PI3K/AKT/mTOR/GSK-3β pathway is one of the most studied molecular signaling routes for the development of new drugs.12 GSK-3β kinase is one of the most important signal transduction pathways in eukaryotic cells and plays a crucial role in the regulation of cell growth, survival, proliferation and metabolism; This relationship may also play a very important role in these parasites, since they also have the two kinases identified in their genome, although their function or interaction has not yet been fully demonstrated.13 The PI3K/AKT/mTOR pathway is essential in human cells for cell migration and its reduced function prevents epithelial mesenchymal transition, cell proliferation and wound healing.14 mTOR forms two unique complexes; mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2), the first complex, controls protein synthesis, followed by cell proliferation, migration, differentiation and survival.15 Apart from the fact that phosphorylated PI3K has effects on mTORC2, other factors may lead to its activation, although this is not completely understood.16 However, AKT kinase is a kinase that controls the activities of GSK-3 and fulfills various cellular functions related to cell cycle progression and survival.17 Deregulated Phosphatidylinositol-3-Kinase (PI3K) generates AKT/mTOR signaling, initiating the development of several diseases including cancer and its progression, obesity, cardiovascular diseases and diabetes.18 In addition to regulating the expression of growth factors including Vascular Endothelial Growth Factor (VEGF), Fibroblast Growth Factor (FGF) and Epidermal Growth Factor (EGF), activated AKT/mTOR promotes cell growth and migration, angiogenesis and collagen synthesis.19 Glycogen Synthase Kinase-3β (GSK-3β), in humans, is a serine/threonine kinase, which has been implicated in the pathogenesis of many cancers, with involvement in the regulation of the cell cycle, apoptosis and the immune response.20 Small molecule GSK-3β inhibitors are currently in clinical investigation.20 Glycogen Synthase Kinase-3 (GSK3) is a ubiquitously expressed and constitutively active serine‑threonine kinase that participates in the regulation of many molecular signaling pathways, some of which have also been implicated in neurodegeneration.21 There are two isoforms of GSK3, GSK3-α and GSK3-β encoded by two different genes.21
In the central nervous system, GSK3-β is the most abundant and its expression levels are known to increase with age. GSK3-β is hyperactive in the brain of patients with Alzheimer's disease and there is convincing evidence that it supports its contribution to the pathology through different mechanisms.22 It has been observed that the deregulation of this kinase affects the metabolism and toxicity of the Aβ peptide to myeloid and tau protein in vitro and in vivo in models of the disease.23 Furthermore, GSK3-β activity has been linked to memory consolidation, neurogenesis, synaptic plasticity, long-term potentiation, and inflammation.23
Small molecule GSK-3β inhibitors are currently in clinical investigation. Tumor sequencing has revealed genomic alterations in GSK-3β, but assessment of the genomic landscape in malignancies is lacking.24 In one study, more than one hundred thousand tumors from two databases were evaluated to analyze GSK-3β alterations (Domoto et al.; 2016).19 GSK-3β expression and immune cell infiltration data were analyzed in all cancer types, and Programmed Death Ligand 1 (PD-L1) expression was compared between GSK-3β-mutation tumors and the wild types, GSK-3β was mutated at a rate of 1 %.19
The main characterized physiological substrate of AKT kinase is Glycogen Synthase Kinase-3β (GSK-3β), which was initially identified as a kinase, which regulates glycogen synthesis in response to insulin.25 GSK-3β is a ubiquitously expressed serine/threonine protein kinase whose activity is inhibited by phosphorylation, for example, it can be inhibited by phosphorylation of Ser-residues in the N-terminal region (Ser21 in GSK3α and Ser9 in GSK3β) through AKT kinase in response to growth factor stimulation.26 In addition to glycogen synthase, GSK-3β phosphorylates a wide range of substrates, including several transcription factors and the translation initiation factor eIF2B.27 Therefore, previous studies have shown that GSK-3β is inhibited as a result of growth factor stimulation and activation of the PI3/AKT/mTOR signaling pathway, in various cell types, such as neurons, epithelial cells, among others.28
Studies carried out show the relationship of the complex molecular mechanisms triggered by the β to myeloid peptide that profoundly affect mitochondrial performance and advocate the inclusion of small molecules directed at the PI3K/GSK-3β axis, which can lead to designing therapies for possible pharmacological interventions against neurodegenerative diseases (Gao et al.; 2011).29
In another study, which analyzed the PI3K/AKT/mTOR and GSK-3B pathway, progress has been seen in the development of immunotherapy techniques to mitigate the development and progression of neoplasms; New data have revealed that GSK-3β kinase and the PI3K/AKT/mTOR pathway represent an important anticancer target, both as a modulator of numerous tumor cell pathways responsible for tumor growth, proliferation, metastasis, and as a regulator of the signaling pathways responsible for the immune response of cells of the innate and adaptive immune system, mainly NK cells and T lymphocytes.30 Therefore, it is important to understand the role of GSK-3 kinase in trypanosomatids and clarify its potential as a new therapeutic target.
Protein kinases in trypanosomatidsTrypanosomatids, like other eukaryotes, have protein kinases, which can regulate these processes by signal transduction events mediated by phosphorylation, controlled by the antagonistic action of protein phosphatases and protein kinases.31 The superfamily of protein kinases in them is composed of Atypical Protein Kinases (aPK) and Eukaryotic Protein Kinases (ePK). Trypanosomatids are evolutionarily divergent eukaryotes and exhibit unique characteristics in their cell cycle, many of which will be regulated by protein kinases.32 This includes its unique apparatus for chromosome segregation and the regulatory processes that govern the highly orchestrated duplication and segregation of single-copy organelles.32 Therefore, it is important to know the state of the art on this topic.32
Recent research has explored the role of individual Leishmania protein kinases in promastigote survival, metacyclic differentiation and amastigote replication, as well as their role in infectivity.33 There are seventeen Mitogen-Activated Protein Kinases (MAPKs) in Leishmania and they were the focus of most of those studies, which highlighted the roles of MPK9 and MPK3 in flagellum maintenance, the latter being phosphorylated by MKK1.33 Another example is the MPK2 kinase, previously shown to modulate Aquaglyceroporin 1 (AQP1) and Amino Acid Transporter (APP3), proteins involved in drug resistance and the response to arginine depletion, respectively, and therefore, the survival of intracellular amastigotes.33 Suppression and persistence of amastigote growth was observed in null mutants of TOR3 and MAPK4, MPK10 which has been implicated in amastigote differentiation and CRK1 and CRK3 in cell cycle control and amastigote survival.33 Only DYRK1 was shown to be essential for differentiation to metacyclic promastigotes, and there is a knowledge gap regarding the role of protein kinases in promastigote to amastigote differentiation, cell infection, and survival in the sandfly mosquito.33
GSK-3/AKT kinases in trypanosomatidsGlycogen Synthase (GSK-3) is a promising kinase of great pharmacological interest by pharmaceutical companies. This kinase has become a new objective of basic research against pathogens that cause parasitic diseases, since orthologous genes of GSK-3 have been identified in their genomes and kinomes. The first, evidence of the relationship in a molecular signaling cascade, between the AKT/GSK-3 kinases, was the phosphorylation of glycogen synthase in rabbit skeletal muscle and subsequently several phosphorylation targets for this kinase were found in the human organism.34
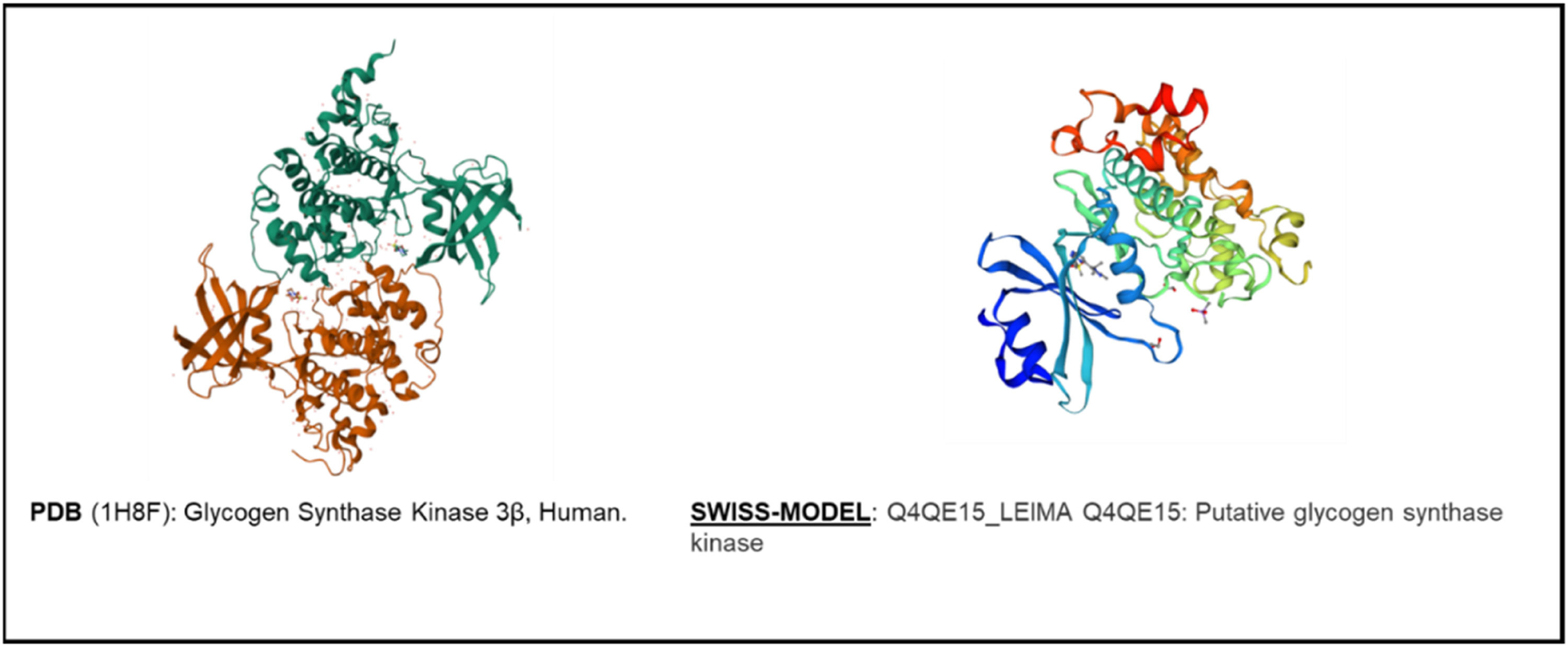
In the genome of trypanosomatids, two GSK-3 proteins are found, very similar in terms of their nucleotide identity, but with different molecular weights at the protein level.35 Various studies point out the importance of obtaining structural information of the AKT/GSK3 pathways, as crucial objectives to carry out in vitro and bioinformatic studies, which lead to the identification of new compounds or for the reuse of drugs, in second uses, against these parasite kinases. Computational methodologies, such as molecular docking, allow rapid screening of potential candidates based on prior knowledge of protein binding sites and active conformations (Ochoa et al.; 2016).36 Furthermore, hybrid methodologies are available to include protein flexibility indirectly, using a molecular dynamics simulation of the target prior to drug screening.37
The development of a safe, efficient and economical effective therapy in the different stages of trypanosomatids is an urgent priority and a historical debt to treat these diseases; Therefore, this GSK-3 kinase is undoubtedly a promising pharmacological target, which could affect different molecular signaling pathways in the complex life cycle of these parasites. Using Interference Techniques (iRNA), it has been observed that T. brucei parasites stop multiplying and their morphology is altered, which demonstrates the importance of the kinase for their survival during the different stages of infection. Since growth arrest after RNA interference appeared to be more profound for T. brucei GSK-3 short (Tb10.161.3140) than for T. brucei GSK-3 long (Tb927.7.2420),38 We can then focus on short GSK-3, which is a kinase with an N-terminal maltose binding fusion that was cloned, expressed and purified in a functional form. The significance of a library of GSK-3-focused inhibitors against the T. brucei recombinant enzyme, short GSK-3, was evaluated to determine whether compounds that inhibit enzymatic activity could also block growth and proliferation of these parasites.38 Among the compounds active against parasites, there was an excellent correlation between T. brucei GSK-3 short kinase inhibiting activity and T. brucei growth inhibition. Therefore, there is reasonable genetic and chemical validation of short GSK-3 as a drug target for T. brucei.39 In the case of Leishmania spp, from a bioinformatic approach to the GSK-3 kinase, it has been crucial to perform a computational screening of new compounds, for repositioning of drugs known to inhibit or modulate its activity.39 Bioinformatics methodologies, such as molecular docking, allow rapid detection of possible candidates based on prior knowledge of protein binding sites and active conformations.40 Furthermore, hybrid methodologies are available to include protein flexibility indirectly using molecular dynamics simulation of the target prior to drug screening. These methods have been previously published and from them it has been possible to select a list of molecules, with promising results against AKT-mTOR kinases.40
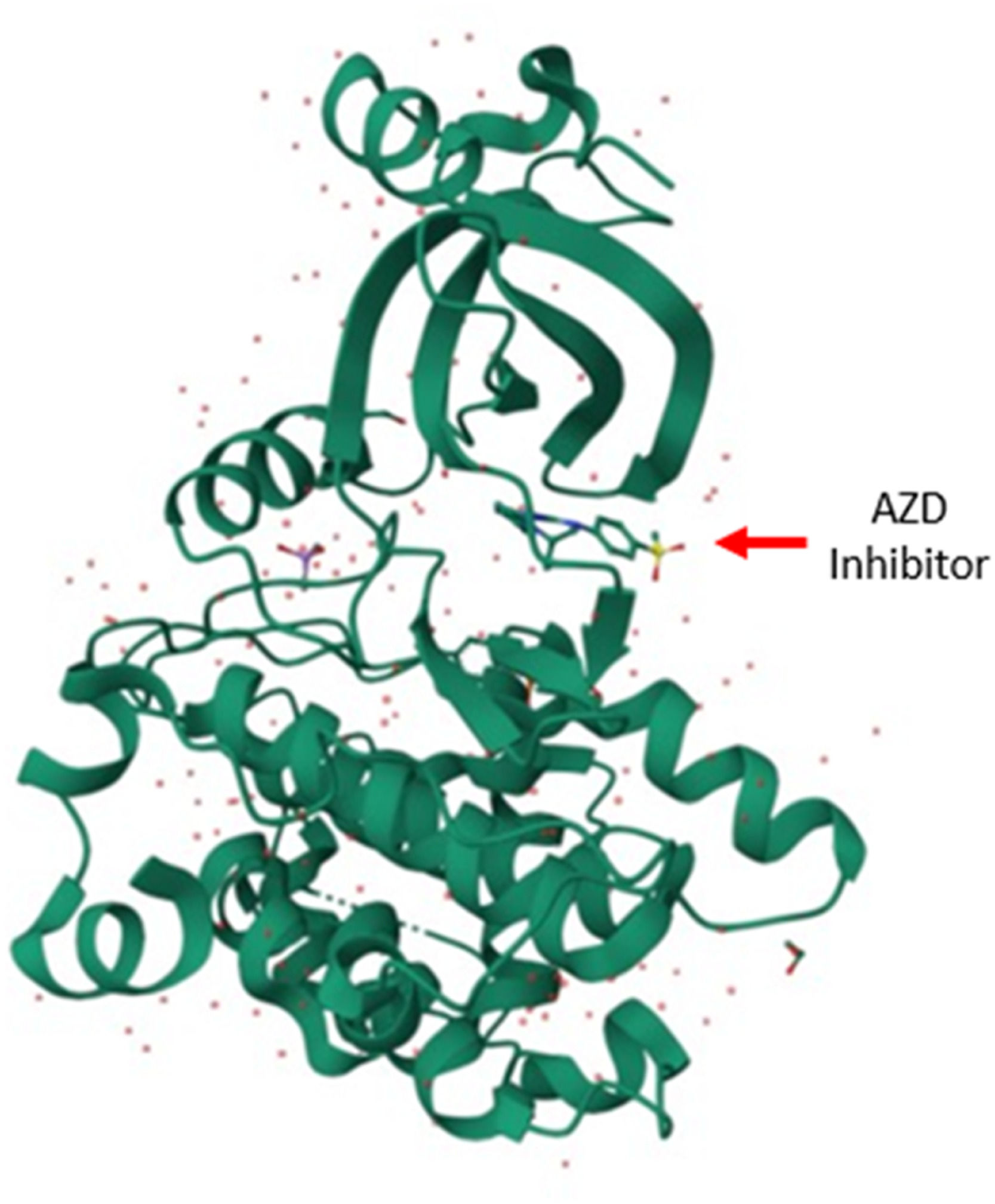
Studies on the parasite Trypanosoma cruzi have shown that it expresses two proteins (short GSK-3 and long GSK-3) with homology to HsGSK-3, it was demonstrated that the protein isoform is essential for the growth of the parasite and its survival (Oduor et al.; 2011). The authors concluded that the evolution of variations conditions the ATP-binding domain of GSK-3.41 In summary, in relation to GSK-3, selective inhibitors of the AKT/GSK-3 pathway can be designed to eliminate the parasite and thus contribute to the control of these diseases41 (Figs. 1 and 2). It is noteworthy that the advent of new antiparasitic agents to overcome the limitations of current pharmacological therapy against leishmaniasis, trypanosomiasis, has fallen on several compounds such as 6-Bromoindirubin-3′-Oxime (6BIO) and several other analogues, 6-substituted protein present in mollusks and plants showing respective inverse selectivity of kinases, most potently targeting the Leishmania Cyclin-dependent Kinase 1 (CDK1) homolog.42
Recently, the AKT/GSK-3 pathway was identified in trypanosomatids as a possible pharmacological target for the treatment of parasitic diseases. In research conducted in China, they demonstrated that GSK-3 was essential for parasite viability, and its inhibition caused cell cycle defects, which included G2/M cell cycle arrest and apoptosis-like death (Xingi et al.; 2009).43 This knowledge is important for the discovery of anti-trypanosomatid drugs, since previous studies demonstrated that the inhibition of GSK-3 leads to non-survival and therefore to an antiparasitic action.43 Therefore, it is expected that this knowledge is crucial for studies of structural relationship and biological activity, which will lead to an adequate understanding of the mechanism of its phosphorylation in the AKT/GSK-3 cell signaling pathway, which apparently is vital for the survival of the parasite. Studies showed through an RNA interference (RNAi) assay and chemical validation that GSK-3 from T. brucei is a promising drug target for the treatment of sleeping sickness in Africa (Ojo et al.; 2008).38 GSK inhibitor interactions, expressed as the calculated interaction energy, could be predicted and improved by using software, which calculates the strength of this interaction. Human GSK-3β and T. brucei short GSK-3 are only 41 % homologous, therefore it seems possible that selective inhibitors against T. brucei short GSK-3 could be found. In fact, molecular modeling studies show differences in the active sites that should be able to translate into the development of selective inhibitors.38
In general, the approach of the studies reviewed and analyzed above should guide us to be able to design new inhibitors against kinases, which can interfere or block specific phosphorylation sites of the AKT/GSK-3 pathway in parasites and be able to avoid adverse effects from their differences when compared to other types of cells. Previous reports have validated (GSK-3) as a pharmacological target against the Leishmania parasite, therefore the evaluation and knowledge of these new routes is of clinical importance; because GSK-3 is involved in a series of signaling pathways associated with the regulation of receptors, cell proliferation, differentiation and cell death. This is why they studied Leishmania donovani GSK-3, which was inhibited by 6‑bromo-5-methylindirubin-3′-oxime, causing deregulation of the cell cycle and induction of apoptosis-like (Martínez et al.; 2020).44 These lethal effects for the parasite were partially rescued by overexpression of the short form of GSK-3, so the enzyme was genetically and pharmacologically validated. The sequence of the short form of GSK-3 is conserved in L. donovani and L. infantum, species that cause the most aggressive form of the visceral leishmaniasis disease; therefore, inhibitors against one species, whether visceral or cutaneous, will presumably be active in the other species, highlighting their intraspecies pharmacological potential. Furthermore, the anti-inflammatory effects found in GSK-3β inhibitors in humans could reduce the inflammatory pathology associated with leishmaniasis.44
Taking into account that current chemotherapy has numerous drawbacks and there is no human vaccine against trypanosomatids, the need to discover new effective drugs is urgent.45 To this end, more and more studies of combinatorial therapy against cutaneous and visceral leishmaniasis are being suggested, while the repurposing of drugs such as Artesunate, an effective drug for the treatment of malaria, is a rational approach to fight the disease. At the same time, many research groups show interest in natural products to discover new compounds, in order to minimize possible side effects. For example, Artesunate, the most stable derivative of the sesquiterpene lactone artemisinin, which is derived from a Chinese plant, appears to possess anti-Leishmania activity and prevent pain and neuroinflammation induced by L. amazonensis in BALB/c mice.46
In recent years, great progress has been made through molecular, genetic and chemical approaches to validate kinases, including (GSK-3), as potential drug targets and study their role in the parasite life cycle. In general, in the center of attention are the members of the RAC and CMGC group, namely Cyclin-Dependent Kinases (CDK), Glycogen Synthase Kinase 3 (GSK-3), AKT-like kinase, Regulated Kinases by Dual-Specificity Tyrosine (DYRK) and Mitogen-Activated Protein Kinases (MAPK).47,51
In Leishmania spp, GSK-3 has been validated by both pharmacological evaluation and genetic manipulation, where Leishmania spp promastigotes were refractory to GSK-3 deletion, these studies suggest that GSK-3 is an essential gene.48 Allosteric inhibition of the kinase resulted in cell cycle deregulation, i.e., G1 phase arrest followed by apoptosis-like death in the parasites, suggesting that GSK-3 may have a direct or indirect role in the progression of the cell cycle. Apart from the essentiality of the kinase, there are additional reasons that make this kinase a good pharmacological target for the design of anti-Leishmania drugs.48
Although most pharmacological compounds are also competitive inhibitors of mammalian GSK-3, the selectivity index between Leishmania and human cell cytotoxicity appears to be high in most cases due to the tolerance of inhibition of host kinase in adult mammals. This redundancy of the mammalian homologous kinase, as well as the aforementioned arguments, highlights the kinase as an excellent candidate for the discovery of new drugs, specifically targeting a non-homologous site of the human GSK-3 kinase39.
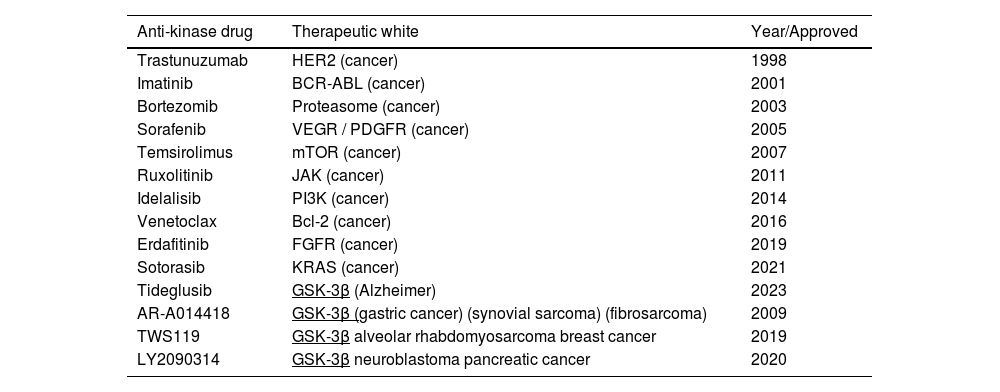
Kinase studies in recent years analyze these proteins as potential participants in the PI3K/AKT signaling pathway. For this purpose, they compared Leishmania kinases with annotated human kinases belonging to signaling pathways where AKT kinase participates (Ochoa et al., 2019).37 Subsequently, models were built and pharmacology metrics were calculated, with a virtual screening approach using molecular docking and a complementary ligand. The drug compounds used were grouped and classified according to their effectiveness against different therapeutic targets.37 In one study, a known human GSK-3β inhibitor found was tested using an experimental approach against the parasite to validate its anti-Leishmania activity (Borba, et al.; 2019). To complement the study, the selected kinases were assigned to a protein-protein interaction network previously constructed for different Leishmania species, to review their validating interactions as well as promising molecular targets in Leishmania.49 In Leishmania spp, genes that may be related to the AKT kinase are expressed, which were cloned and sequenced, finding a new gene, called Ld-RAC/AKT, from Leishmania donovani (MHOM/IN/80Dd8), which encoded a protein closely related to putative serine‑RAC/AKT (Varela et al.; 2017). The data from this study show that the Ld-RAC/AKT-like protein can behave as a survival molecule in Leishmania parasites and could become a new target for leishmaniasis therapy as it is possibly related to the interaction of AKT/GSK-3 in these parasites, similarly to what occurs in humans (Table 1).50,51
Approved anti-kinase drugs and their therapeutic targets.
However, due to the few discoveries of new drugs for these parasites and the urgent need to obtain new drugs with known mechanisms of action to treat these forgotten and neglected diseases; it is then proposed to study in depth the essential kinase GSK-3 s and its counterpart the kinase AKT in the different species of trypanosomatids. It should be remembered that the inhibition of GSK-3 s stops the cell cycle in G1 and generates apoptosis-like in these parasites. Additionally, it has specific changes in its amino acid sequence, strategically located in the ATP binding pocket, compared to human GSK-3β, among these changes Gln 185 h, Leu132h GSK-3β in humans vs. His 155 Ld are observed, Met 100 Ld GSK-3 in Leishmania, these and other molecular differences can be explored to design new drugs that are more effective and specific in all trypanosomatids that have these orthologous genes. It is important to remedy the historical debt with these forgotten and neglected diseases, in relation to the discovery and development of new drugs that can improve the quality of life of these patients.
ConclusionForgotten and neglected parasitic diseases, urgently need new medications for their treatment and control. Many of the current medications are resistant and their mechanisms of action are poorly understood. Therefore, the discovery of new kinases in the different trypanosomatids will allow work on specific therapeutic targets, through different strategies such as computational screening, reducing costs that have always been a barrier to the discovery of new drugs against parasites. The GSK- kinase has been shown to be essential in these parasites and therefore it is important to develop new drugs through different strategies that allow the development of an allosteric inhibitor against its main domains. Kinases and their molecular signaling pathways are little studied and have high pharmacological potential, as has been demonstrated in other models such as cancer.
This research has been funded by the General Direction of Research at Universidad Santiago de Cali, under call No 07–2022 and 01-2024.