Bloodstream infections (BSI) are severe diseases associated with high mortality rates, longer length of hospital stay, and elevated costs. The spread of resistant microorganisms has become a public health problem. For this reason, surveillance measures have become a recognized need, since they can help healthcare teams to monitor the occurrence of nosocomial infections and to identify the common causative microorganisms.1 The purpose of this study was to assess the frequency and the antimicrobial resistance profiles of pathogens recovered from blood cultures in one public and one private hospital from Niterói city, Rio de Janeiro, Brazil.
Patients with bloodstream infection from one 227-bed public general hospital and one 123-bed private general hospital from August 2009 to August 2010 were identified. In both hospitals, blood samples were cultured by automated methods (BacT/ALERT, bioMérieux Clinical Diagnostics – Marcy l’Etoile, France). Microbiological identification and antimicrobial susceptibility testing were performed in the public institution using the Vitek 2 automated system (bioMérieux). In the private hospital, the organisms were identified using Enterokit B and Enterokit C (PROBAC do Brasil Produtos Bacteriológicos Ltda. – São Paulo, Brazil). Antimicrobial susceptibility testing was performed by disk diffusion method.2Enterobacteriaceae were tested for carbapenemase production using the modified Hodge test. E. coli, K. pneumoniae, K. oxytoca and P. mirabilis isolates were tested for extended spectrum beta lactamase (ESBL) production. Staphylococcus aureus isolates were submitted to cefoxitin disk diffusion test to confirm oxacillin resistance.2 Differences between intensive care unit (ICU) and wards of each hospital were assessed for statistical significance using the chi-squared test or Fisher's exact test. The significance level was set at p ≤ 0.05.
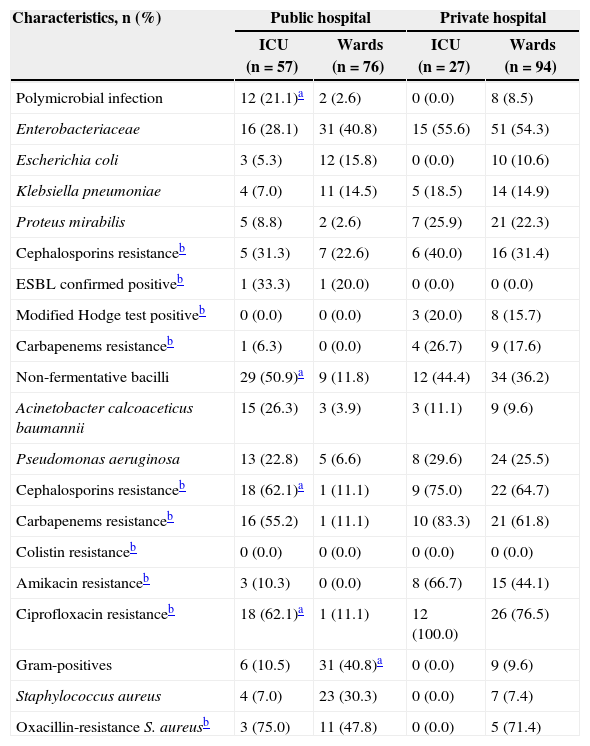
A total of 234 BSI episodes (one episode per patient) were detected, yielding 254 isolates. There were 22 (7.5%) polymicrobial episodes, the majority (n = 14, 63.6%) found in the public hospital. Most of the BSI episodes were caused by Enterobacteriaceae (n = 113, 46.5%), followed by non-fermentative Gram-negative bacilli (n = 84, 34.6%). Enterobacteriaceae were prevalent among isolates recovered from patients of wards in both hospitals (n = 31, 40.8%; n = 51, 54.3%). The production of ESBL was investigated; despite the Latin America SENTRY program having shown that the rates of ESBL-producing Enterobacteriaceae, especially K. pneumoniae and E. coli, are among the highest in the world,3 only one strain of E. coli was identified to be ESBL-producer in the wards of the public hospital, and only one strain of K. pneumoniae in the ICU of the same hospital. Carbapenems are used to treat severe infections caused by multidrug-resistant organisms, especially by ESBL-producing pathogens. During the last decade, carbapenem resistance has emerged among clinical isolates of Enterobacteriaceae, and this has been increasingly attributed to the production of β-lactamases able to hydrolyze these agents.4 We investigated carbapenemase production among Enterobacteriaceae isolates and found, in the private hospital, ten strains of K. pneumoniae and one strain of Klebsiella sp. positive (Table 1).
Characteristics of bacterial isolates recovered from blood cultures in participating public and private hospitals.
| Characteristics, n (%) | Public hospital | Private hospital | ||
|---|---|---|---|---|
| ICU (n = 57) | Wards (n = 76) | ICU (n = 27) | Wards (n = 94) | |
| Polymicrobial infection | 12 (21.1)a | 2 (2.6) | 0 (0.0) | 8 (8.5) |
| Enterobacteriaceae | 16 (28.1) | 31 (40.8) | 15 (55.6) | 51 (54.3) |
| Escherichia coli | 3 (5.3) | 12 (15.8) | 0 (0.0) | 10 (10.6) |
| Klebsiella pneumoniae | 4 (7.0) | 11 (14.5) | 5 (18.5) | 14 (14.9) |
| Proteus mirabilis | 5 (8.8) | 2 (2.6) | 7 (25.9) | 21 (22.3) |
| Cephalosporins resistanceb | 5 (31.3) | 7 (22.6) | 6 (40.0) | 16 (31.4) |
| ESBL confirmed positiveb | 1 (33.3) | 1 (20.0) | 0 (0.0) | 0 (0.0) |
| Modified Hodge test positiveb | 0 (0.0) | 0 (0.0) | 3 (20.0) | 8 (15.7) |
| Carbapenems resistanceb | 1 (6.3) | 0 (0.0) | 4 (26.7) | 9 (17.6) |
| Non-fermentative bacilli | 29 (50.9)a | 9 (11.8) | 12 (44.4) | 34 (36.2) |
| Acinetobacter calcoaceticus baumannii | 15 (26.3) | 3 (3.9) | 3 (11.1) | 9 (9.6) |
| Pseudomonas aeruginosa | 13 (22.8) | 5 (6.6) | 8 (29.6) | 24 (25.5) |
| Cephalosporins resistanceb | 18 (62.1)a | 1 (11.1) | 9 (75.0) | 22 (64.7) |
| Carbapenems resistanceb | 16 (55.2) | 1 (11.1) | 10 (83.3) | 21 (61.8) |
| Colistin resistanceb | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Amikacin resistanceb | 3 (10.3) | 0 (0.0) | 8 (66.7) | 15 (44.1) |
| Ciprofloxacin resistanceb | 18 (62.1)a | 1 (11.1) | 12 (100.0) | 26 (76.5) |
| Gram-positives | 6 (10.5) | 31 (40.8)a | 0 (0.0) | 9 (9.6) |
| Staphylococcus aureus | 4 (7.0) | 23 (30.3) | 0 (0.0) | 7 (7.4) |
| Oxacillin-resistance S. aureusb | 3 (75.0) | 11 (47.8) | 0 (0.0) | 5 (71.4) |
Prevalence: number of resistant isolates divided by the number of isolates tested. ICU, intensive care unit. Resistance to third- and/or fourth-generation cephalosporins. Screening and confirmatory tests for extended-spectrum beta lactamases-ESBL (CLSI, 2010). Modified Hodge test was performed for Enterobacteriaceae (CLSI, 2010). Carbapenems resistance to meropenem and/or imipenem.
The non-fermentative bacilli were the major cause of bacteremia in the ICU of the public hospital. In the private hospital, non-fermentative bacilli were mostly recovered from wards, Pseudomonas aeruginosa being the principal agent of infection. Data collected by the Latin American SENTRY Program showed that the prevalence of isolates resistant to all antimicrobial agents, except polymyxins, has been continuously increasing, and P. aeruginosa resistance rates were slightly higher among isolates recovered in Brazil, compared to other areas.3 All non-fermentative bacilli were susceptible to colistin in both hospitals. In our study, 23.1% of the strains recovered from the public hospital ICU were resistant to meropenem. In the private hospital, 87.5% and 60.9% of strains recovered from ICU and wards, respectively, were resistant to this antibiotic (Table 1).
Other studies have reported S. aureus as the major cause of bloodstream infection in Brazil, South America, and North America.3 The pattern of resistance observed in our research was similar to other Brazilian hospitals where MRSA accounted for 31.0% to 66.7% of the isolates (Table 1).5
Etiology and antimicrobial susceptibility patterns of microorganisms recovered from blood culture varied between inpatient units, pointing out the need for continuous surveillance, since local data is essential to improve therapeutic options, as well to prevent and control infections.
Conflict of interestAll authors declare to have no conflict of interest.
This research was supported by the Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Pró-Reitoria de Pesquisa, Pós-Graduação e Inovação da UFF (PROPPI/UFF).