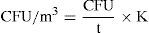An aeromycological study verifies the presence and quantifies the concentration of fungal propagules in the air. It is very important in the hospital setting because of the increasing numbers of immunosuppressed and severely ill patients. The objective of this study was to determine the concentration of fungi in the air of the intensive care unit (ICU) of “Dr. Manuel Gea González” General Hospital.
MethodsThis is a descriptive, observational cross-sectional study. Air samples were obtained with a single stage Thermo-Andersen Viable Particle Sampler (Thermo Electron Corporation - Massachusetts, U.S.A.) in a Petri dish with potato dextrose agar for 15minutes at two different times (morning and afternoon) and heights (1 and 1.5 meters). The Petri dishes were incubated for five to seven days at 27° C, the number of colonies was counted, and the total CFU/m3 was determined. The isolated fungal genera were identified by morphological features. Epi Info v. 3.4.3 © was used for statistical analysis.
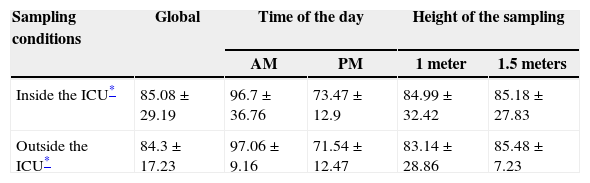
ResultsThe mean concentration of fungi in the air of the ICU was 85.08 ± 29.19 CFU/m3; while in the outside air it was 84.3 ± 17.23 CFU/m3 (p = 0.96). The fungi isolated were: Cladosporium spp., Penicillium spp., Aspergillus spp. (non-fumigatus), Fusarium spp., Exophiala spp., Syncephalastrum spp., and Acremonium spp.
DiscussionFungal spores were found in the air of the ICU and Cladosporium spp. was the most frequently isolated fungi. There was no difference according to sampling time or height.
Bioaerosols are aerial suspensions of particles from live organisms, microorganisms or other biological materials.1–3 Aeromycology is the branch of aerobiology that studies the dispersion of spores and other fungal elements in indoor and outdoor air, the changes in their concentrations, and the factors that affect those changes.3 Fungal spores enter hospitals through ventilation systems and fungi develop on multiple surfaces, releasing more spores.1,3 The number of spores in indoor air varies depending on climate, weather, air currents, humidity, temperature, time of the day, type and maintenance of ventilation systems, age of the buildings, movement of people, cleaning, and the presence of plants or food.1,3–7
Immunosuppressed patients with severe neutropenia, chronic granulomatous disease, and acquired immunodeficiency syndrome (AIDS) have the highest risk of developing invasive fungal infection.8–10 The main fungal genera related with these diseases are Aspergillus, Candida, Fusarium, Penicillium, Mucor, and Rhizopus.11–17 Invasive aspergillosis (especially by A. fumigatus), candidemia, disseminated fusariosis, infections by P. marneffei, and zygomycosis have a mortality rate that can reach 100%.10–17
The single stage Thermo-Andersen Viable Particle Sampler (Thermo Electron Corporation – Massachusetts, USA) is the most frequently used air sampler.8,18,19 In the detection phase, the amount of colony forming units (CFU) per cubic meter of air is determined, and then the identification of the fungi is performed by culture or by molecular techniques.7,9,18–21
Because the number of immunosuppressed and severely ill patients is increasing worldwide, especially in the hospital setting, this study was designed to determine the concentration of fungi in the air of the intensive care unit (ICU) of this hospital.
Materials and methodsThis was a descriptive, observational, cross-sectional study. The universe was the air of the ICU of “Dr. Manuel Gea Gonzalez” General Hospital. The ICU is divided in different sections with different air volumes: five single rooms (30 m3 each), one double room (120 m3), one aisle (67.5 m3), and one nurse module (37.5 m3). For convenience, an air sample was taken from the area just outside the ICU (one meter away from the entrance door), from one single room (picked randomly), from the double room, from the aisle, and from the nurse module. The total air volume of the areas that were sampled inside the ICU was 255m3. The air volume sampled from the five selected areas (inside and outside the ICU) was 0.1415m3.14 The unit is ventilated by a central air conditioning unit without fans or open windows. The unit can accommodate up to seven patients and always has at least ten staff members working. The highest level of activity occurs during morning hours. Among the studied variables are: humidity, temperature, CFU per air volume, and fungal agents.
Air samples were obtained with a single stage Thermo-Andersen Viable Particle Sampler (Thermo Electron Corporation – Massachusetts, USA) in a Petri dish with potato dextrose agar. The sampler was placed in the center of each room, and each sample was collected with a vacuum flow of 28.3 liters/min for 15minutes.14,22 In each sampling area, four samples were collected: one at each time period (morning and afternoon) and one at each height (one and 1.5 meters). Humidity and temperature were measured with a hygrothermograph (Control Company – Friendswood, Texas, USA). Nobody was allowed in or out of the room during sampling.
After sampling, the Petri dishes were incubated for five to seven days at 27° C.7,14 After that, the number of colonies was counted in every Petri dish. The total number of fungal colonies present in the ICU was determined by obtaining the mean of the colony count in the four areas sampled. This count was stratified by time and height of the sample. The concentration of fungal propagules in the air was expressed in CFU/m3. For its calculation, a correction factor was used for the colony count, based on the probability that more than one viable propagule could have passed through the same hole and impacted the culture medium. The formula used was:
Where CFU was the corrected colony count, N was the number of holes in the perforated plate of the sampler (400), and P was the number of colonies that grew in the medium.
The corrected count represents the real number of CFUs present in the sampled air. After that, the CFU/m3 of air was determined with the following formula:
Where CFU was the corrected colony count, t was the total sampling time expressed in minutes, and K was a conversion factor from cubic feet to cubic meters (K = 35).
The identification of fungi was performed by observation of the macroscopic characteristics of the colonies and the microscopic characteristics of the sporulating hyphae.5
The statistical analysis was performed with the Epi Info v. 3.4.3© software, and the means and standard deviations of humidity, temperature, and colony forming units per air volume were determined according to sampling time and height. Independent Student's t-test was used to determine whether there was a statistically significant difference between the means of CFU per air volume according to sampling area, time, and height. Differences were considered significant when the p-value was below 0.05.
All procedures performed were in accordance with and approved by the ethical standards of the “Dr. Manuel Gea Gonzalez” General Hospital Review Board and Ethics Committee. The principles of the Helsinki Declaration of 1975 with the modifications of 1983, and the Mexican General Health Law were followed.
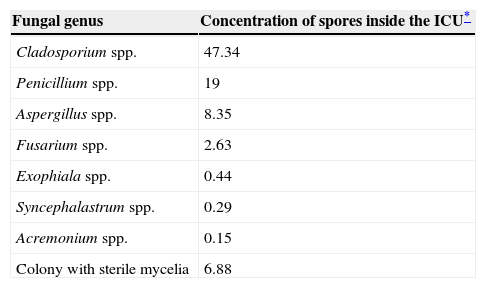
ResultsThere was no difference in the fungal spore concentration of the air inside the ICU when compared with the air outside the unit (Table 1). These concentrations were not affected by the time of sampling (p = 0.13 and p = 0.14, respectively) or the height of sampling (p = 0.99 and p = 0.92, respectively) (Table 1). Several colonies were cultured, and the fungal genera isolated were: Cladosporium spp., Penicillium spp., Aspergillus spp. (non-fumigatus), Fusarium spp., Exophiala spp., Syncephalastrum spp. and Acremonium spp. (Table 2). The mean humidity during sampling was 38.88 ± 3.20% and the mean temperature was 24.09 ± 0.82° C.
Fungal spore concentrations in the air according to different sampling conditions.
Fungal genera isolated.
| Fungal genus | Concentration of spores inside the ICU* |
|---|---|
| Cladosporium spp. | 47.34 |
| Penicillium spp. | 19 |
| Aspergillus spp. | 8.35 |
| Fusarium spp. | 2.63 |
| Exophiala spp. | 0.44 |
| Syncephalastrum spp. | 0.29 |
| Acremonium spp. | 0.15 |
| Colony with sterile mycelia | 6.88 |
In the present study, the mean concentration of fungi in the air of the ICU was 85.08 ± 29.19 CFU/m3. This result is similar to that obtained by a study performed in the pulmonology ward at a hospital in Lublin, Poland, where the annual fungal concentration varied from 9.9 to 96.1 CFU/m3.21 No difference was found in this study when the fungal concentration in the air from inside the ICU was compared with the air from outside the unit (p = 0.96). The present findings differ from the results of a study conducted at a university hospital in Rotterdam, Netherlands, where the spore concentration was higher in open areas inside the hospital when compared to the hematology ward.17
No differences were found in the concentration of spores in the ICU according to the time of sampling (p = 0.13). This result contrasts with the previously mentioned Polish study, where the concentration of fungal spores was significantly higher in the morning than in the afternoon (p < 0.01).21
The fungal genera isolated in the present study were: Cladosporium spp., Penicillium spp., Aspergillus spp., Fusarium spp., Exophiala spp., Syncephalastrum spp. and Acremonium spp. None of the fungal colonies isolated in this study corresponded to Aspergillus fumigatus. The present results are in accordance with the results of other aeromycology studies. An aeromycology study performed in the ICUs and operating rooms of a hospital in the city of Araraquara, Sao Paulo, Brasil, found that the most isolated fungal genera were Cladophialophora spp., Fusarium spp., Penicillium spp., Chrysosporium spp. and Aspergillus spp.5 A study performed in the waiting room of the allergy service of the Infanta Cristina Hospital in Spain showed that most of the fungal spores belonged to the genus Cladosporium (C. cladosporioides and C. herbarum).6 A research project performed in the ICUs, operating rooms, biomedical laboratories, and lobbies of five general hospitals in Seoul, South Korea, found that the most frequently isolated fungal genera in the air were: Cladosporium spp. (30%), Penicillium spp.(20%-25%) and Aspergillus spp. (15%-20%).7 A study conducted at the transplantation ward of a hospital in Bogota, Colombia, found a mean of 2.8 CFU/L of Aspergillus.19
The presence of the fungi that were isolated from the air of the present ICU, especially Aspergillus spp. and Fusarium spp., is worrisome because the patients of these units are usually severely ill or immunosuppressed, and this is the group at the highest risk of suffering from potentially fatal invasive fungal diseases.10,12,13 However, some of these fungal genera also represent a threat to immunocompetent hosts, such as the healthcare staff of the ICU. Fungal spores of several genera, such as Cladosporium spp., have been related to asthma exacerbation.3Aspergillus spp. has been associated with several diseases such as rhinosinusitis, chronic cavitary pulmonary aspergillosis, aspergilloma, allergic bronchopulmonary aspergillosis, and skin and wound infections, among others.3,23 Meanwhile, spores of Aspergillus spp., Fusarium spp., Cladosporium spp., and Penicillium spp. can produce mycotoxin-related disease.9,18
The present results underline the importance of establishing control measures to improve air quality inside the ICU, aiming to reduce fungal-related morbidity and mortality. Among these measures, the following can be mentioned: periodic measurements of fungal propagules in the air with aeromycological studies, rigorous cleaning with disinfectants and dust removal, routine equipment maintenance, humidity control, air filtration with high efficiency particle air filters, and use of laminar flow ventilation systems, among others.1,4,24
ConclusionFungal spores were found in the air of the ICU (85.08 ± 29.19 CFU/m3), and Cladosporium spp. was the most isolated fungi. There was no difference according to sampling time or height.
Conflict of interestAll authors declare to have no conflict of interest.
To the staff of the research unit and the ICU of “Dr. Manuel Gea Gonzalez” General Hospital, and to Dr. Yamilett Morales, for their collaboration.