Toxicity is the most frequently reported reason for modifying or discontinuing the first combined antiretroviral therapy regimens, and it can cause significant morbidity, poor quality of life and also can be an important barrier to adherence, ultimately resulting in treatment failure and viral resistance. Elderly patients with HIV/AIDS (≥50 years) may have a different profile in terms of treatment modification due to higher incidence of comorbidities and polypharmacy. The aim of this study was to describe the incidence of modifying or discontinuing first combined antiretroviral therapy regimen due to toxicity (TOX-MOD) during the first year of treatment at the IPEC – FIOCRUZ HIV/AIDS cohort, Rio de Janeiro, Brazil, stratified by age. Demographic, clinical and treatment characteristics from antiretroviral-naïve patients who first received combined antiretroviral therapy between Jan/1996 and Dec/2010 were collected. Incidence rate and confidence interval of each event were estimated using quasipoisson model. To estimate hazard ratio (HR) of TOX-MOD during the first year of combined antiretroviral therapy Cox's proportional hazards regression was applied. Overall, 1558 patients were included; 957 (61.4%), 420 (27.0%) and 181 (11.6%) were aged <40, 40–49, and ≥50 years, respectively. 239 (15.3%) events that led to any modifying or discontinuing within the first year of treatment were observed; 228 (95.4%) of these were TOX-MOD, corresponding to an incidence rate of 16.6/100 PY (95% CI: 14.6–18.9). The most frequent TOX-MOD during first combined antiretroviral therapy regimen were hematologic (59; 26.3%), central nervous system (47; 20.9%), rash (42; 19.1%) and gastrointestinal (GI) (38; 16.7%). In multivariate analysis, incidence ratio of TOX-MOD during the first year of combined antiretroviral therapy progressively increases with age, albeit not reaching statistical significance. This profile was maintained after adjusting the model for sex, combined antiretroviral therapy regimen and year of combined antiretroviral therapy initiation. These results are important because not only patients are living longer and aging with HIV, but also new diagnoses are being made among the elderly. Prospective studies are needed to evaluate the safety profile of first line combined antiretroviral therapy on elderly individuals, especially in resource-limited countries, where initial regimens are mostly NNRTI-based.
The introduction of highly active antiretroviral therapy (HAART) during the 1990s was crucial to reduce HIV related morbidity and mortality rates turning HIV infection into a chronic condition. In Brazil, where HAART has been universally available for more than 15 years, prolonged survival has been shown.1,2 Currently, with more than 220,000 patients receiving combined antiretroviral therapy (cART), Brazil is in a unique position to evaluate treatment outcomes of cART in the context of developing countries.
Several studies from developed and developing countries have investigated the rates and reasons for modification or discontinuation of the first cART regimen, and their results indicate that up to 69% of patients may modify their regimen over time; 25–44% of them in the first 12 months of treatment.3–19 The most frequently reported reason for modifying the first cART has been treatment-associated toxicity5–8,10,12,13,17,19–24 that can cause significant morbidity, poor quality of life and can also be an important barrier to adherence,16,25 ultimately resulting in treatment failure and viral resistance.26 We have previously described that, in our cohort, toxicity was the main reason for modifying or discontinuing (MOD) the first HAART regimen.5
Elderly patients (≥50 years old) with HIV/AIDS may have a different profile in terms of treatment modification due to higher incidence of comorbidities and polypharmacy.27 Also, the general characteristics of aging may have considerable influence on the pharmacokinetics of medications. These changes can result in increased antiretroviral (ARV) concentrations, which may lead to higher risk of related toxicity28 and increased rates of treatment modifications related to toxicities.22
This study describes the incidence of MOD the first cART regimen due to toxicity during the first year of treatment at the Evandro Chagas Clinical Research Institute, Oswaldo Cruz Foundation (IPEC – FIOCRUZ) HIV/AIDS cohort for patients who started cART in five different age groups (18–29, 30–39, 40–49, 50–59, ≥60 years).
Materials and methodsDescription of the clinical cohort and study populationThis study was conducted at the IPEC/FIOCRUZ where care has been provided to HIV/AIDS patients since 1986. A longitudinal observational clinical database has been maintained on patients receiving HIV care at IPEC. Cohort procedures have been described and results published elsewhere.29–31 Briefly, data are updated regularly using outpatient and inpatient clinical documentation and laboratory testing results. Prescription of ARV therapy (drug, dates of use, and dose) is documented by the medical provider and support staff in the clinical records. Trained abstractors record the information onto standardized forms for processing.
For this study, we included data from 1558 antiretroviral (ART)-naïve patients who first received cART between January 1996 and December 2010, with follow-up through August 2011. The IPEC Institutional Review Board has reviewed and approved the study.
Study definitionsAge at HAART initiation was the variable of interest across all analyses. Patients were stratified as 18–29 years and 30–39 years (“younger”), 40–49 years (“older”); 50–59 years and ≥60 years (“elderly”). “Elderly” was defined according to CDC definition for HIV/AIDS patients.32 Other variables used to describe our cohort included demographic, clinical and treatment related characteristics.
HIV exposure categories were presented as: heterosexual (women and men separately); men who have sex with men (MSM); injecting drug users (IDU), and others (not specified). Race was grouped as white and non-white. Schooling was stratified in ≤4 years; 5–8 years; 9–11 years; >11 years. Starting cART while participating in an ART naive clinical trial, baseline CD4+T lymphocyte count (cells/μL), baseline HIV viral load (log10copies/mL) and AIDS-defining disease were also accessed.
cART was defined as two NRTIs in combination with at least one PI or one NNRTI. Patients were grouped according to the year of cART initiation before and after year 2004, when new, less toxic and friendlier ARV options became available. First cART regimens were defined as PI-based regimen (with or without booster), NNRTI-based regimen and others. PI-based regimen that used ritonavir (RTV) as booster and the most frequent first cART regimens were also recorded.
For this study, we have only assessed cART modifications or discontinuations related to toxicity (TOX-MOD) that occurred during the first year after treatment initiation. cART discontinuation related to toxicity was defined as treatment interruption caused by any ARV-related toxicity. cART modification due to toxicity was defined as a toxicity driven substitution of at least one ARV in the regimen. ARV dosage adjustments were not considered as modifications.
The type and date of TOX-MOD were defined as given in the medical chart, and were grouped as follows: hematologic (anemia, thrombocytopenia, leukopenia, pancytopenia), central nervous system (CNS) (neuropsychiatric manifestation, e.g. hallucinations, vertigo, insomnia, nightmares, depression, phobia), peripheral neuropathy (PN), rash, GI (nausea, vomiting, diarrhea), liver (liver enzymes increase, hyperbilirubinemia, jaundice), renal (creatinine clearance decrease, serum creatinine increase, proteinuria, lithiasis, acute renal failure) and metabolic (dyslipidemia and lipodystrophy).
Statistical analysisThe outcomes of interest were overall toxicity and the most frequent toxicities during the first year after treatment initiation that had led to cART MOD grouped as described above. We estimated the incidence rate and confidence interval (95% CI) of each event stratified by age groups (18–29, 30–39, 40–49, 50–59, ≥60 years) using quasipoisson model and reported it as the number of occurrences per 100 persons-years (PY). Deaths and MOD related to other reasons during the first year of ART were censored at the time of their occurrence. Patients who did not MOD their first ART were censored at one year after cART initiation.
Cox's proportional hazards regression was applied to estimate the HR of overall TOX-MOD during the first year of cART according to the clinical/demographic characteristics. The model was also adjusted for toxicity risk factors previously identified (sex, type of regimen, and year of treatment initiation).5 The proportional hazard assumption was tested by Schoenfeld residuals analysis.
We used the statistical software R, version 2.14.1 (www.r-project.org) for all statistical analyses.
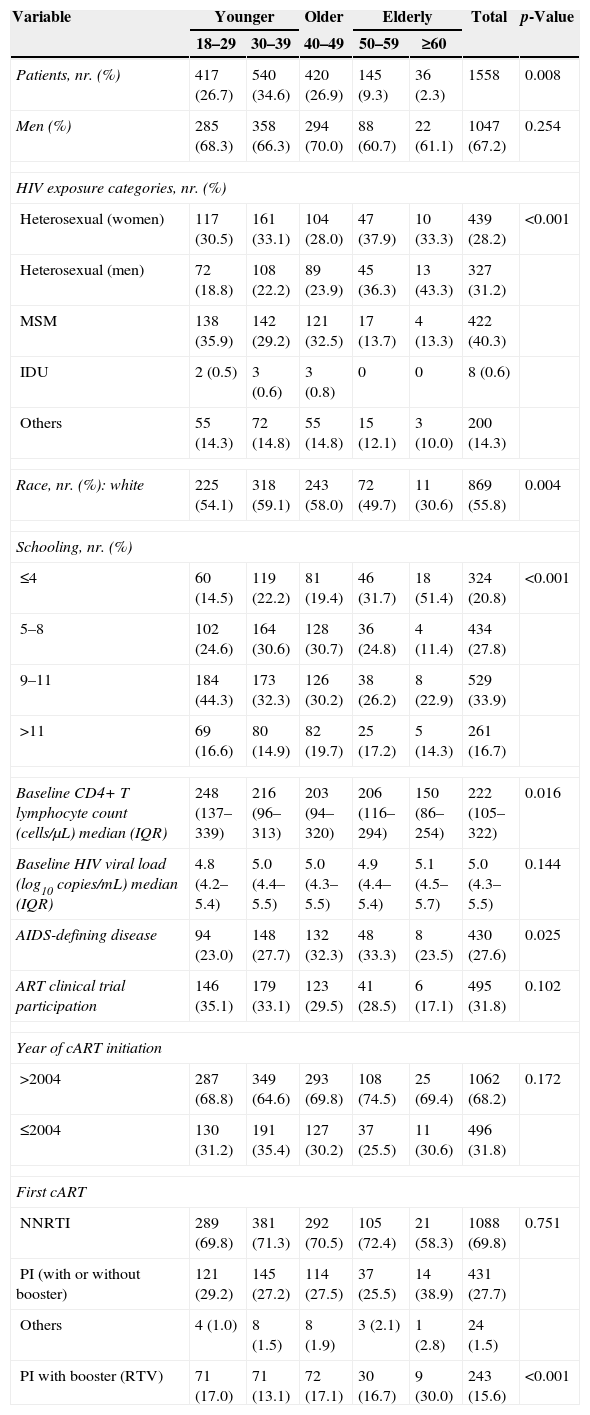
ResultsDemographic data and treatment characteristicsSelected demographic values and treatment characteristics distributed according to the decade of life are summarized in Table 1. A total of 1558 ART naïve patients were included in this analysis. At cART initiation, 957 (61.4%) were younger (<40 years), 420 (27.0%) were older (40–49 years), and 181 (11.6%) were elderly (≥50 years). The median age was 36 years [interquartile range (IQR): 29–43].
Demographics, clinical, and HIV treatment characteristics of individuals at IPEC – FIOCRUZ HIV/AIDS cohort stratified by age at cART initiation.
| Variable | Younger | Older | Elderly | Total | p-Value | ||
|---|---|---|---|---|---|---|---|
| 18–29 | 30–39 | 40–49 | 50–59 | ≥60 | |||
| Patients, nr. (%) | 417 (26.7) | 540 (34.6) | 420 (26.9) | 145 (9.3) | 36 (2.3) | 1558 | 0.008 |
| Men (%) | 285 (68.3) | 358 (66.3) | 294 (70.0) | 88 (60.7) | 22 (61.1) | 1047 (67.2) | 0.254 |
| HIV exposure categories, nr. (%) | |||||||
| Heterosexual (women) | 117 (30.5) | 161 (33.1) | 104 (28.0) | 47 (37.9) | 10 (33.3) | 439 (28.2) | <0.001 |
| Heterosexual (men) | 72 (18.8) | 108 (22.2) | 89 (23.9) | 45 (36.3) | 13 (43.3) | 327 (31.2) | |
| MSM | 138 (35.9) | 142 (29.2) | 121 (32.5) | 17 (13.7) | 4 (13.3) | 422 (40.3) | |
| IDU | 2 (0.5) | 3 (0.6) | 3 (0.8) | 0 | 0 | 8 (0.6) | |
| Others | 55 (14.3) | 72 (14.8) | 55 (14.8) | 15 (12.1) | 3 (10.0) | 200 (14.3) | |
| Race, nr. (%): white | 225 (54.1) | 318 (59.1) | 243 (58.0) | 72 (49.7) | 11 (30.6) | 869 (55.8) | 0.004 |
| Schooling, nr. (%) | |||||||
| ≤4 | 60 (14.5) | 119 (22.2) | 81 (19.4) | 46 (31.7) | 18 (51.4) | 324 (20.8) | <0.001 |
| 5–8 | 102 (24.6) | 164 (30.6) | 128 (30.7) | 36 (24.8) | 4 (11.4) | 434 (27.8) | |
| 9–11 | 184 (44.3) | 173 (32.3) | 126 (30.2) | 38 (26.2) | 8 (22.9) | 529 (33.9) | |
| >11 | 69 (16.6) | 80 (14.9) | 82 (19.7) | 25 (17.2) | 5 (14.3) | 261 (16.7) | |
| Baseline CD4+ T lymphocyte count (cells/μL) median (IQR) | 248 (137–339) | 216 (96–313) | 203 (94–320) | 206 (116–294) | 150 (86–254) | 222 (105–322) | 0.016 |
| Baseline HIV viral load (log10copies/mL) median (IQR) | 4.8 (4.2–5.4) | 5.0 (4.4–5.5) | 5.0 (4.3–5.5) | 4.9 (4.4–5.4) | 5.1 (4.5–5.7) | 5.0 (4.3–5.5) | 0.144 |
| AIDS-defining disease | 94 (23.0) | 148 (27.7) | 132 (32.3) | 48 (33.3) | 8 (23.5) | 430 (27.6) | 0.025 |
| ART clinical trial participation | 146 (35.1) | 179 (33.1) | 123 (29.5) | 41 (28.5) | 6 (17.1) | 495 (31.8) | 0.102 |
| Year of cART initiation | |||||||
| >2004 | 287 (68.8) | 349 (64.6) | 293 (69.8) | 108 (74.5) | 25 (69.4) | 1062 (68.2) | 0.172 |
| ≤2004 | 130 (31.2) | 191 (35.4) | 127 (30.2) | 37 (25.5) | 11 (30.6) | 496 (31.8) | |
| First cART | |||||||
| NNRTI | 289 (69.8) | 381 (71.3) | 292 (70.5) | 105 (72.4) | 21 (58.3) | 1088 (69.8) | 0.751 |
| PI (with or without booster) | 121 (29.2) | 145 (27.2) | 114 (27.5) | 37 (25.5) | 14 (38.9) | 431 (27.7) | |
| Others | 4 (1.0) | 8 (1.5) | 8 (1.9) | 3 (2.1) | 1 (2.8) | 24 (1.5) | |
| PI with booster (RTV) | 71 (17.0) | 71 (13.1) | 72 (17.1) | 30 (16.7) | 9 (30.0) | 243 (15.6) | <0.001 |
IQR, interquartile range.
Each age category had more male (overall average of 67.2%), and the number of white individuals decreased with age (54.1% for 18–29 years and 30.6% for ≥60 years; p<0.0043). HIV exposure categories significantly fluctuated over age groups (p<0.001). Considering only men, the proportion of MSM was: 48.4%, 39.7%, 41.2%, 19.3% and 18.3% for 18–29 years, 30–39 years, 40–49 years, 50–59 years and ≥60 years, respectively (p<0.0001). Elderly patients had less years of education (≤4 years) than younger and older patients (14.5% for 18–29 years and 51.4% for ≥60 years; p<0.0001). There was no statistically significant difference on ART clinical trial participation among the age groups.
At the time of cART initiation, 430 (27.6%) patients had already presented at least one AIDS-defining disease, and the frequency increased with age, except for patients ≥60 years (p=0.0247). Baseline CD4+T lymphocyte count significant decreased with age (248cells/μL for 18–29 years and 150cells/μL for ≥60 years; p=0.016), while there was no significant difference on baseline HIV viral load among the age groups.
The majority of patients started cART after 2004 (overall average 68.2%); as well as the majority of patients started the first cART with a NNTRI-based regimen (1088; 69.8%) and there were no statistically significant differences among age groups neither for the calendar year nor for the regimen. RTV-boosted PI-based regimen was used, by 56.4% (243/431) of patients and it significantly increased with age (17.0% for 18–29 years and 30.0% for ≥60 years; p<0.0001).
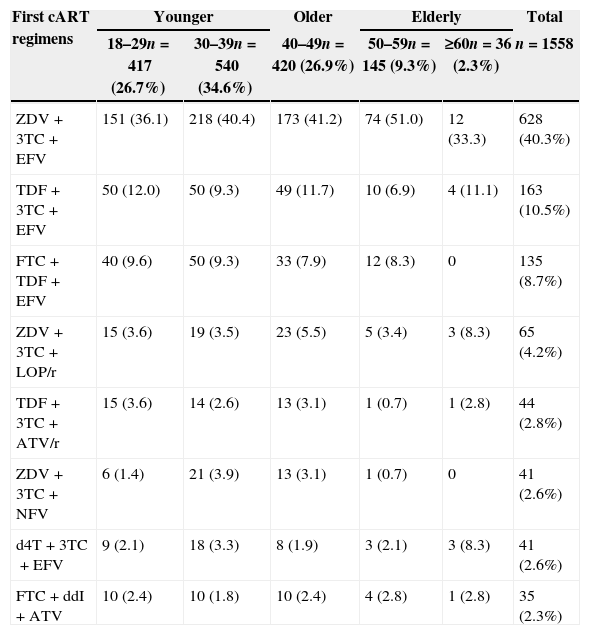
The most frequent first cART regimens stratified by age are depicted in Table 2. A combination of zidovudine (ZDV)+lamivudine (3TC)+efavirenz (EFV) was used by two-fifths of the study population (628, 40.3%). Nevirapine (NVP) was used in 3.8% of cART regimens. Comparing NRTI use along the calendar year, TDF use increased after 2004, while the use of d4T, ddI, and ABC decreased. A continuous increase on ZDV and 3TC use was observed from 1996 to 2010.
Most frequent first cART regimens at IPEC – FIOCRUZ HIV/AIDS cohort stratified by age at cART initiation.
| First cART regimens | Younger | Older | Elderly | Total | ||
|---|---|---|---|---|---|---|
| 18–29n=417 (26.7%) | 30–39n=540 (34.6%) | 40–49n=420 (26.9%) | 50–59n=145 (9.3%) | ≥60n=36 (2.3%) | n=1558 | |
| ZDV+3TC+EFV | 151 (36.1) | 218 (40.4) | 173 (41.2) | 74 (51.0) | 12 (33.3) | 628 (40.3%) |
| TDF+3TC+EFV | 50 (12.0) | 50 (9.3) | 49 (11.7) | 10 (6.9) | 4 (11.1) | 163 (10.5%) |
| FTC+TDF+EFV | 40 (9.6) | 50 (9.3) | 33 (7.9) | 12 (8.3) | 0 | 135 (8.7%) |
| ZDV+3TC+LOP/r | 15 (3.6) | 19 (3.5) | 23 (5.5) | 5 (3.4) | 3 (8.3) | 65 (4.2%) |
| TDF+3TC+ATV/r | 15 (3.6) | 14 (2.6) | 13 (3.1) | 1 (0.7) | 1 (2.8) | 44 (2.8%) |
| ZDV+3TC+NFV | 6 (1.4) | 21 (3.9) | 13 (3.1) | 1 (0.7) | 0 | 41 (2.6%) |
| d4T+3TC+EFV | 9 (2.1) | 18 (3.3) | 8 (1.9) | 3 (2.1) | 3 (8.3) | 41 (2.6%) |
| FTC+ddI+ATV | 10 (2.4) | 10 (1.8) | 10 (2.4) | 4 (2.8) | 1 (2.8) | 35 (2.3%) |
3TC, lamivudine; ATV/r, atazanavir/ritonavir; d4T, stavudine; ddI, didanosine; EFV, efavirenz; FTC, emtricitabine; LOP/r, lopinavir/ritonavir; NFV, nelfinavir; TDF, tenofovir; ZDV, zidovudine.
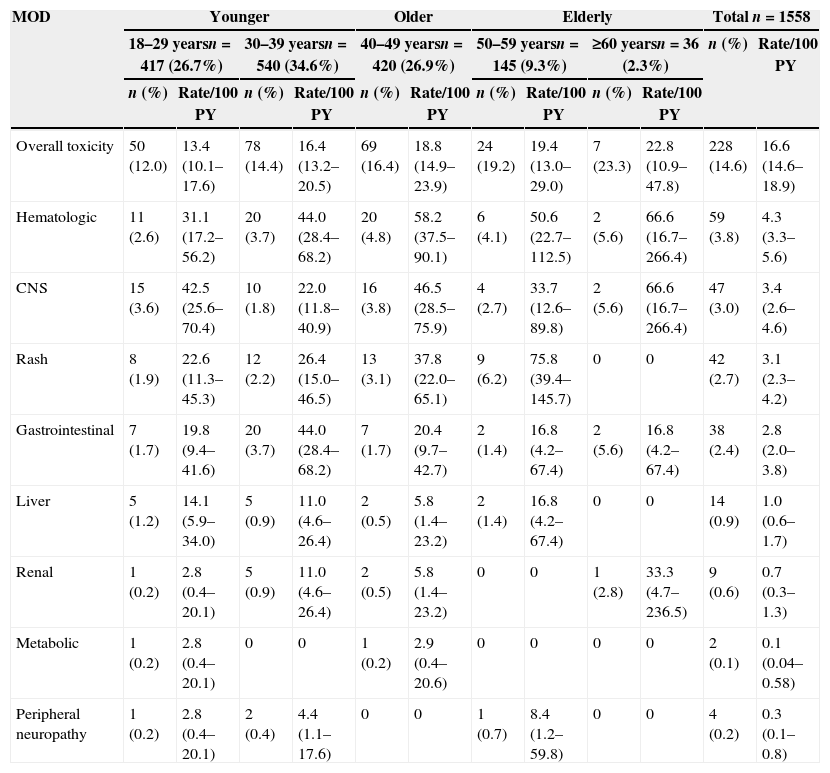
Patients were followed for a total of 1369 PY, from ART initiation up to one year of treatment or up to any MOD that occurred within the first year of treatment. A total of 239 (15.3%) events that led to any MOD within the first year of treatment were observed; 228 (95.4%) of these were related to toxicity (TOX-MOD), corresponding to an incidence rate of 16.6 per 100 PY (95% CI: 14.6–18.9). The median time from ART initiation to TOX-MOD during the first year of ART was 1.46 months (IQR: 0.5–4.0). The overall probability of TOX-MOD in the first year of ART was 14.6% (228/1558). Almost half of the patients who presented TOX-MOD were on ZDV+3TC+EFV.
The most frequent toxicity events associated with MOD during the first cART regimen were hematologic (59/228; 26.3%), CNS (47/228; 20.9%), rash (42/228; 19.1%), and GI (38/228; 16.7%). The great majority of the hematologic events were anemia (48; 81.4%), followed by leucopenia (6; 10.3%). For the GI events, the most common were nausea and vomiting (25; 65.8%), gastrointestinal intolerance (7; 18.4%) and diarrhea (4; 10.5%).
Frequency and incidence of TOX-MOD increased with age (Table 3). For younger patients the overall frequency of TOX-MOD was 12.0% and 14.4% (18–29 and 30–39 years, respectively) while for older and elderly patients increases of 2% and 3% per decade of age were observed, respectively. The incidence rate of TOX-MOD for patients aged 18–29 years was 13.4 per 100 PY (95% CI: 10.1–17.6) while for patients aged 50–59 years, and ≥60 years was 19.4 per 100 PY (95% CI: 13.0–29.0) and 22.8 per 100 PY (95% CI: 10.9–47.8), respectively. Stratifying by age groups, both frequency and incidence increased with age for most of toxicities, and this increase was more pronounced from 40 to 49 years and above. In contrast, the incidence rate of TOX-MOD by gastrointestinal events was much higher among patients aged 30–39 years (44.0 per 100 PY (95% CI: 28.4–68.2). Frequency of liver, renal, PN and metabolic toxicities was low in this study.
Incidence rate of TOX-MOD on the first cART regimen at IPEC – FIOCRUZ HIV/AIDS cohort stratified by age at cART initiation.
| MOD | Younger | Older | Elderly | Total n=1558 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18–29 yearsn=417 (26.7%) | 30–39 yearsn=540 (34.6%) | 40–49 yearsn=420 (26.9%) | 50–59 yearsn=145 (9.3%) | ≥60 yearsn=36 (2.3%) | n (%) | Rate/100 PY | ||||||
| n (%) | Rate/100 PY | n (%) | Rate/100 PY | n (%) | Rate/100 PY | n (%) | Rate/100 PY | n (%) | Rate/100 PY | |||
| Overall toxicity | 50 (12.0) | 13.4 (10.1–17.6) | 78 (14.4) | 16.4 (13.2–20.5) | 69 (16.4) | 18.8 (14.9–23.9) | 24 (19.2) | 19.4 (13.0–29.0) | 7 (23.3) | 22.8 (10.9–47.8) | 228 (14.6) | 16.6 (14.6–18.9) |
| Hematologic | 11 (2.6) | 31.1 (17.2–56.2) | 20 (3.7) | 44.0 (28.4–68.2) | 20 (4.8) | 58.2 (37.5–90.1) | 6 (4.1) | 50.6 (22.7–112.5) | 2 (5.6) | 66.6 (16.7–266.4) | 59 (3.8) | 4.3 (3.3–5.6) |
| CNS | 15 (3.6) | 42.5 (25.6–70.4) | 10 (1.8) | 22.0 (11.8–40.9) | 16 (3.8) | 46.5 (28.5–75.9) | 4 (2.7) | 33.7 (12.6–89.8) | 2 (5.6) | 66.6 (16.7–266.4) | 47 (3.0) | 3.4 (2.6–4.6) |
| Rash | 8 (1.9) | 22.6 (11.3–45.3) | 12 (2.2) | 26.4 (15.0–46.5) | 13 (3.1) | 37.8 (22.0–65.1) | 9 (6.2) | 75.8 (39.4–145.7) | 0 | 0 | 42 (2.7) | 3.1 (2.3–4.2) |
| Gastrointestinal | 7 (1.7) | 19.8 (9.4–41.6) | 20 (3.7) | 44.0 (28.4–68.2) | 7 (1.7) | 20.4 (9.7–42.7) | 2 (1.4) | 16.8 (4.2–67.4) | 2 (5.6) | 16.8 (4.2–67.4) | 38 (2.4) | 2.8 (2.0–3.8) |
| Liver | 5 (1.2) | 14.1 (5.9–34.0) | 5 (0.9) | 11.0 (4.6–26.4) | 2 (0.5) | 5.8 (1.4–23.2) | 2 (1.4) | 16.8 (4.2–67.4) | 0 | 0 | 14 (0.9) | 1.0 (0.6–1.7) |
| Renal | 1 (0.2) | 2.8 (0.4–20.1) | 5 (0.9) | 11.0 (4.6–26.4) | 2 (0.5) | 5.8 (1.4–23.2) | 0 | 0 | 1 (2.8) | 33.3 (4.7–236.5) | 9 (0.6) | 0.7 (0.3–1.3) |
| Metabolic | 1 (0.2) | 2.8 (0.4–20.1) | 0 | 0 | 1 (0.2) | 2.9 (0.4–20.6) | 0 | 0 | 0 | 0 | 2 (0.1) | 0.1 (0.04–0.58) |
| Peripheral neuropathy | 1 (0.2) | 2.8 (0.4–20.1) | 2 (0.4) | 4.4 (1.1–17.6) | 0 | 0 | 1 (0.7) | 8.4 (1.2–59.8) | 0 | 0 | 4 (0.2) | 0.3 (0.1–0.8) |
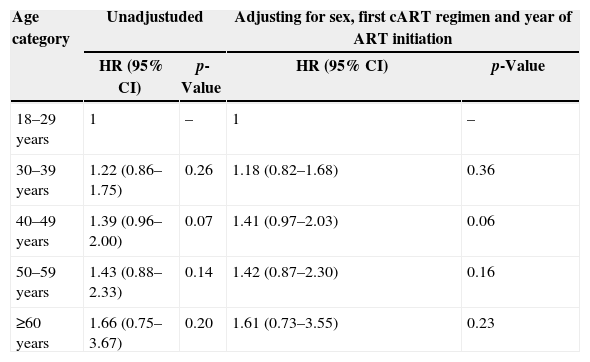
The results of the multivariate model (Cox's proportional hazards regression) showed that the incidence ratio of TOX-MOD during the first year of cART progressively increased with age, albeit not reaching statistical significance. This profile was maintained after adjusting for sex, cART regimen and year of cART initiation: HR 1.18 (95% CI: 0.82–1.68) for 30–39 years; HR 1.41 (95% CI: 0.97–2.03) for 40–49 years; HR 1.42 (95% CI: 0.87–2.30) for 50–59 years; HR 1.61 (95% CI: 0.73–3.55) for ≥60 years (Table 4). No violation of Schoenfeld proportional hazard assumption was found.
Hazard ratio (HR) and 95% confidence interval (95% CI) estimated by Cox proportional hazards regression of TOX-MOD on first cART at IPEC – FIOCRUZ HIV/AIDS cohort stratified by age at cART start.
| Age category | Unadjustuded | Adjusting for sex, first cART regimen and year of ART initiation | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| 18–29 years | 1 | – | 1 | – |
| 30–39 years | 1.22 (0.86–1.75) | 0.26 | 1.18 (0.82–1.68) | 0.36 |
| 40–49 years | 1.39 (0.96–2.00) | 0.07 | 1.41 (0.97–2.03) | 0.06 |
| 50–59 years | 1.43 (0.88–2.33) | 0.14 | 1.42 (0.87–2.30) | 0.16 |
| ≥60 years | 1.66 (0.75–3.67) | 0.20 | 1.61 (0.73–3.55) | 0.23 |
Our results provide important insights into the toxicities that led to first line cART MOD during the first year of treatment as a function of age at cART initiation among patients followed at a clinical research institute in a middle-income country. Roughly 95% of the first ART regimen MOD was related to toxicity. Hematologic, CNS, rash and GI were the most frequently reported causes of TOX-MOD. These results are important because not only patients are living longer and aging with HIV, but also new diagnoses are being made among the elderly.33–35 In a previous assessment on the incidence of MOD of the first cART regimen in our cohort, evaluating 670 patients who started cART between 1996 and 2006, toxicities within the first year of treatment were observed in 26.7%, corresponding to an incidence rate of 24 per 100 PY (95% CI: 20.0–28.0),5 much higher than the observed in this study (14.6%). This difference can be attributed to the larger number of patients in our cohort who started therapy after 2006, when the use of friendlier, less toxic NRTIs and PIs have dramatically increased, when compared to the initial HAART period until 2006. The same profile of TOX-MOD during the first year of treatment was found in the Caribbean, Central and South America Network for HIV Research (CCASAnet) cohort, with adverse events prompting ART regimen change in 14.4% of patients initiating a HAART regimen, among six of the seven participating clinical sites. Similar to what was found in our cohort, in this multicenter Latin America cohort, hematologic events, 70% of which anemia, were the most frequently observed toxicity.6
Until very recently in 2012, the Brazilian ARV guidelines preferential option for first cART regimen consisted of ZDV+3TC+EFV.36 This may explain the toxicity profile observed, with hematologic and GI events as the most commonly reported, probably associated with ZDV, and CNS and rash events, commonly related to EFV.
The high use of LPV/r and ATV/r as second options for first-line regimen can also explain the high frequency of GI toxicities. However, these observations are based on previous studies, and this study focused on the overall incidence of toxicities stratified by age rather than class-related (NRTI, NNRTI, PI) or even ARV-related toxicities. The use of PI with booster increased with age (Table 1) and no association with calendar year was identified. We noticed that at the time of cART initiation, almost 30% of our patient population had already presented at least one AIDS-defining disease, and this frequency increased with age. Furthermore, CD4+T lymphocyte depletion significantly increased with age. It is well known that clinicians tended to prescribe more PI based regimens with booster for individuals with more advanced immunosuppression, and this may have had an impact on the ARV prescription pattern in our cohort. It could also be the case that patients and their providers could have feared EFV related CNS toxicity among older individuals, and thus PI-based regimens with booster were more prescribed among these patients.
A high incidence of CNS related toxicities on individuals aged 18–29 years (42.5 per 100 PY; 95% CI: 25.6–70.4) when compared to those aged 30–39 years (22.0/100 PY; 95% CI: 11.8–40.9) was observed. Data on the use of recreational drugs were not available for our cohort. We can speculate that a higher use of such drugs among young HIV-infected individuals in our study population could be contributing to this finding. Interactions between agents commonly prescribed for patients with HIV and recreational drugs can occur. Clinicians should encourage open dialog with their patients on this topic, to avoid the risk of drug toxicity.37 Further studies on this topic should be encouraged.
Metabolic, liver, renal and PN related toxicities are more common on the long term, and this could explain the low frequency observed up to one year of cART, which precluded us to compare the frequency and incidence differences of these events among the age groups.
In the multivariate model adjusted for sex, first cART regimen and calendar year of cART initiation, the HR for TOX-MOD increased with age, although this effect did not reach statistical significance. The limited number of patients on the elderly group, especially ≥60 years, could have influenced these results.
Increased risk of toxicity related to cART among elderly individuals was previously observed.38–40 However, very limited data comparing TOX-MOD of first cART among different age groups have been reported, and the few comparisons available41–43 were done between two major age groups only (<50 years and ≥50 years, elderly). Moreover, previous analysis neither for Latin America nor for developing countries from other regions was found. In a study from the Italian Cohort Naïve Antiretrovirals (ICoNA) a significantly higher risk of TOX-MOD on elderly was observed, and this difference in the significance may be attributed to the higher number of elderly in comparison with our population (<50 years, n=4818; ≥50 years, n=399).41 In a cohort from France, TOX-MOD of first cART regimens was independently associated with age and occurs at earlier stages of treatment in individuals ≥50 years. Consistent with our findings, a higher frequency of CNS and hematologic events on elderly people was observed.42
Other authors have also studied the impact of age on TOX-MOD but due to the different methodology and definitions applied, comparisons are difficult. In a study from the UK, MOD for reasons other than virological failure during the first year of cART was higher in those aged <30 years and ≥50 years. Although TOX-MOD was not studied separately, a higher frequency of laboratory abnormalities among the elderly population, specially a decrease in hemoglobin count, could be associated with this finding.43
Recently published results from the PEARLS study have shown that a regimen consisting of tenofovir (TDF)+emtricitabine (FTC)+EFV has shown a better safety profile than ZDV+3TC+EFV, with less hematologic and CNS related toxicities, and can be potentially a better regimen for the elderly individuals.44
Elderly individuals are prone to develop other clinical conditions typical of an aging population, and the medications used to treat such comorbidities may interact with ARV drugs leading to a higher incidence of toxicity. As elderly individuals have been shown to be more adherent to therapy related45 toxicities may also be more frequently given higher cumulative exposure to the drugs.
Our study has limitations. The retrospective nature of the data collection process implies that biases may have influenced our results. Also, we have not assessed toxicities related to cART that did not result in MOD, toxicity grading and patient's adherence level.
Prospective studies are needed to evaluate the safety profile of first line cART on elderly individuals, especially in resource-limited countries, where initial regimens are mostly NNRTI based.
Conflicts of interestThe authors declare no conflicts of interest.