Staphylococcus aureus infections remain associated with considerable morbidity and mortality in both hospitals and the community. There is little information regarding the role of ovarian hormones in infections caused by S. aureus. The aim of this study was to evaluate the effects of ovariectomy in the immune response induced by S. aureus.
MethodsFemale mice BALB/c were ovariectomized (OVX) to significantly reduce the level of ovarian hormones. We also used sham-operated animals. The mice were inoculated intraperitoneally with S. aureus. Blood samples were collected for leukocyte count and bacterial quantification. The uterus and spleen were removed and weighed to calculate the uterine and splenic indexes. Lungs were removed and fractionated for immunohistochemical analysis for macrophage detection (anti-CD68) and relative gene expression of IL-6, IL-1β and TNF-α by RT-PCR.
ResultsOvariectomy enlarged spleen size and generally increased circulating lymphocytes. OVX females experienced a continuation of the initial reduction of lymphocytes and a monocyte and neutrophil late response compared to shams (p≥0.05). Moreover, OVX females showed neutropenia after 168h of infection (p≥0.05). Macrophage response in the lungs were less pronounced in OVX females in the initial hours of infection (p≥0.01). OVX females showed a higher relative gene expression of IL-1β, IL-6 and TNF-α in the lung at the beginning of the infection compared to sham females (p≥0.01). Among the uninfected females, the OVX control females showed a higher expression of IL-6 in the lung compared to the sham control females (p≥0.05). In this model, the lack of ovarian hormones caused a minor increase in circulating leukocytes during the initial stage of infection by S. aureus and increased pulmonary gene expression of IL-1β, IL-6, and TNF-α. Ovariectomy alone enlarged the spleen and increased circulating lymphocytes. Ovarian hormones acted as immunoprotectors against S. aureus infection.
The multiple differences between sexes at all levels of the biological organization suggests that observations about men cannot be generalized indiscriminately to women and vice versa.1 However, the influence of sex hormones (and sex) on disease mechanisms has been poorly studied.2 Sex differences and variations in the severity of inflammatory diseases have been related to levels of female sex hormones, suggesting that these hormones modulate the inflammatory response.3 Sex steroids and immunity are intimately linked, and their mutual regulation is involved in maintaining the immune balance.4 Currently, there has been a growing interest in the immune-endocrine system, including the relationship between sex, sex hormones and their effects on pathophysiological parameters and immune response in adverse conditions.5
Staphylococcus aureus is a major pathogen that can cause a broad spectrum of serious infections including skin infections, pneumonia, and sepsis6 and is notorious for its ability to acquire and/or develop resistance to antibiotics. This attribute, coupled with the high burden of S. aureus infections is a challenge for treatment.7 Infections caused by S. aureus remain associated with considerable morbidity and mortality in both hospitals and the community.8
Experimental and clinical studies indicate sex-specific differences in infectious diseases.9–11 Thus, the present study was performed to evaluate the effects of ovariectomy on the immune response induced by intraperitoneal inoculation of Staphylococcus aureus in female mice.
Materials and methodsStaphylococcus aureusThe reference strain used in this study was Staphylococcus aureus ATCC 25923. We used BHI (Brain Heart Infusion - Becton Dickinson, Heidelberg, Germany) and mannitol salt agar (Becton Dickinson, Heidelberg, Germany) to activate, cultivate, and subculture S. aureus strains for subsequent inoculation in mice. Further, Gram stain, catalase test, coagulase test and PCR (nuc gene) were performed to confirm the identity and purity of the bacterial strain.12 The S. aureus inoculum was obtained by direct suspension, removing three to five colonies (same morphological type) of the plates containing the reference strain. Then, the colonies were transferred to tubes containing sterile saline solution 0,85%, vortexed, and analyzed in a spectrophotometer (Jenway, London, England) to obtain the following parameters: 0.135Abs (660nm), equivalent to 1×108 CFU (Colony Forming Units).13
MiceWe used 80 specific-pathogen-free (SPF) BALB/c female mice aged six to eight weeks from the Multidisciplinary Center for Biological Research in the Area of Science of Animal Laboratory at the State University of Campinas (CEMIB/UNICAMP). The animals were maintained under controlled light conditions (light on from 7am to 7 pm) and temperature (23±3°C), with free access to water and food. All experiments were conducted in accordance with internationally accepted principles for the use and care of laboratory animals as set out in the Animal Research: Reporting of in vivo Experiments (ARRIVE) guidelines and were carried out in accordance with the European Community guidelines (EEC Directive 1986/86/609) and executed after approval by the Animal Ethics Committee (AEC) of the IMS/UFBA, under protocol nº 03/2013.
OvariectomyIn order to significantly decrease the level of sex hormones, female mice were ovariectomized (or sham-operated for control group). The animals were anesthetized with ketamine (Syntec, São Paulo, Brazil) and xylazine (Syntec, São Paulo, Brazil) at doses of 5mg/kg and 50mg/kg, respectively. The surgery was performed according to the current prescribed method in endocrinology.14 After surgery, the animals received prophylactic antibiotic treatment with enrofloxacin 2.5% (Syntec, São Paulo, Brazil) in a single dose of 2.5mg/kg.
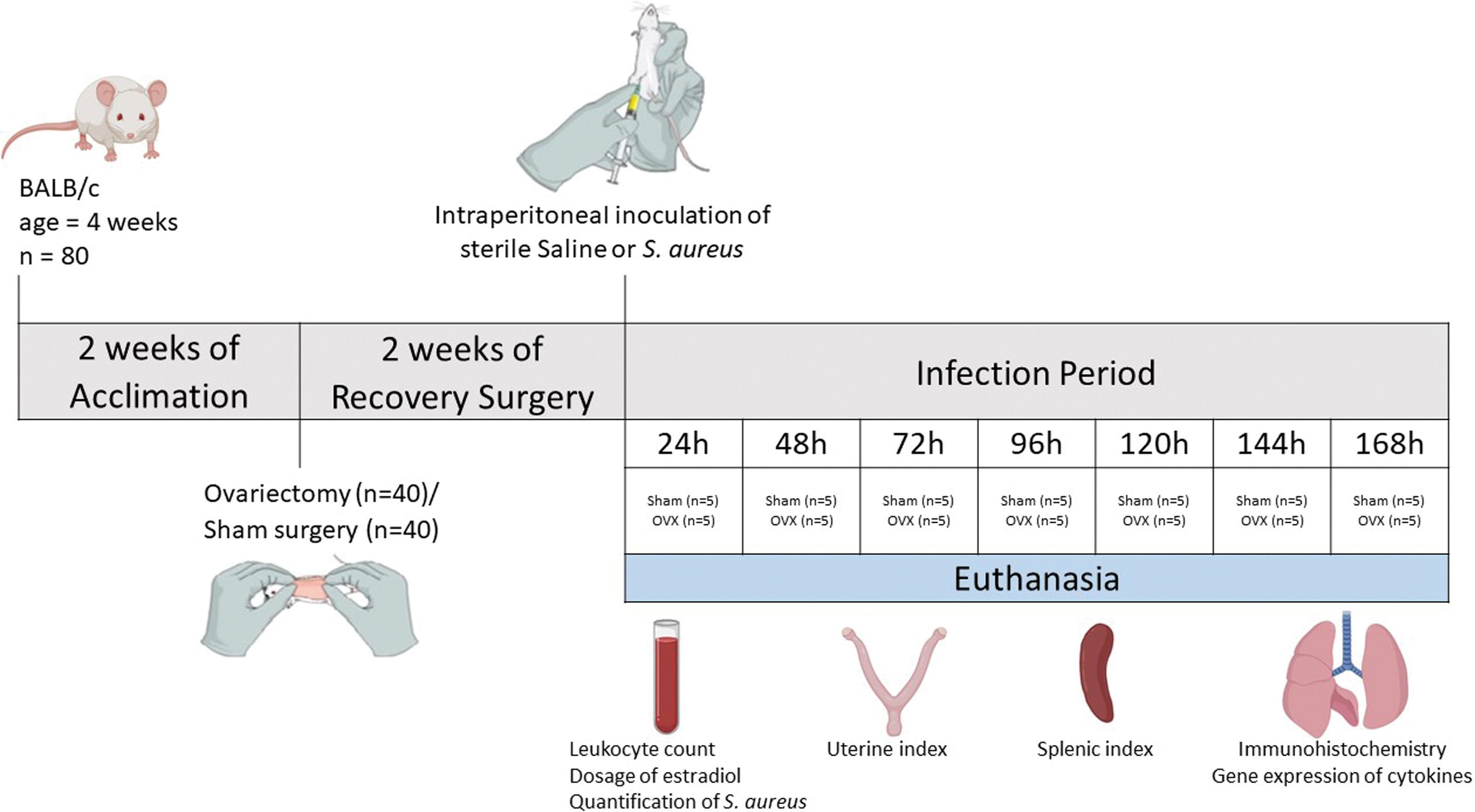
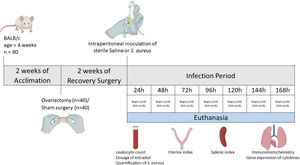
Experimental modelAfter two weeks of ovariectomy, the female mice were inoculated intraperitoneally with sterile saline (control group) or Staphylococcus aureus (experimental groups). The experimental groups were identified according to the time of infection, with seven defined time points: 24, 48, 72, 96, 120, 144 and 168h. Each group consisted of five ovariectomized females (OVX) and five shams. Thus, the infected groups were: Sham 24h, OVX24h, Sham 48h, OVX48h, Sham 72h, OVX72h, Sham 96h, OVX96h, Sham 120h, OVX120h, Sham 144h, OVX144h, Sham 168h, OVX168h. The control group also consisted of five ovariectomized females (OVX control) and five shams (Sham control). Each animal in the control group received 100μl of sterile saline and the infected group received 100μl of S. aureus inoculum (1×108 CFU). At the end of each period of infection (24, 48, 72, 96, 120, 144 and 168h), the animals were euthanized by decapitation. Control group animals were euthanized at 168h. Blood samples were collected for leukocyte count, dosage of serum levels of estradiol using ELISA and quantification of S. aureus by Quantitative Real-time Polymerase Chain Reaction (qPCR). The uterus and spleen were removed and weighed to calculate the uterine index and splenic index, respectively. To calculate the index, the weight of the organ was corrected by the weight of the animal (Amaral et al., 2016). Lungs were removed and fractionated to perform histopathological analysis, immunohistochemistry, and gene expression by Reverse Transcription Quantitative Real-time Polymerase Chain Reaction (RT-qPCR). To histopathological analysis and immunohistochemistry, the tissue was fixed in methacarn solution13,15 (Fig. 1).
Leukocyte countImmediately after euthanasia, 20μl of blood (collected in EDTA tube – BD, São Paulo Brazil) was mixed with 380μl of Turk liquid (NewProv, Pinhais, Brazil) for 20min. Thereafter, the sample was transferred to a Neubauer chamber and analyzed under an optical microscope (Olympus BX51, Japan) to perform a white blood cell count in a magnification ×400 using the four sides of the camera reticles for counting. For the differential count, 100 leukocytes were counted in a blood smear stained with dye panoptic (NewProv, Pinhais, Brazil) through optical microscope using immersion objective. We used the longitudinal method and the values were recorded in percentages (%). Based on the overall count, it was possible to calculate the absolute values for each type of white blood cell count.13 All experiments were performed in triplicate.
Blood DNA extraction and quantification of S. aureus in bloodThe DNA was extracted from blood clots according to the kit protocol Invisorb® Spin Blood Midi Kit (STRATEC Biomedical AG, Birkenfeld, Germany). qPCR was performed in duplicate, using the StepOne Plus Applied Biosystems platform (Thermo Scientific, São Paulo, Brazil). The technique was performed by using a TaqMan probe, using an amplification-based protocol. This protocol includes, in a final volume of 12.5μl of reaction per well, 6.25μl of Master Mix (Thermo Scientific, São Paulo, Brazil), 0.56μl of LTnucF primer (AAATTACATAAAGAACCTGCGACA), 0.56μl of LTnucR primer (GAATGTCATTGGTTGACCTTTGTA) (20pmol), 0.38μl of probe (10mM), 3.5μl of water and 1.25μl of DNA.16 The amplification parameters were: 50°C for one minute, 95°C for 10min followed by 45 cycles of 95°C for 15s and 59°C for one minute. Quantitation was performed using the absolute quantization technique, based on a predetermined standard curve ranging from 107 to 10 microorganisms/μl. For a good analysis, the following characteristics were considered: r2≥0.950 and efficiency between 95% to 105%. All experiments were performed in triplicate with three independent repetitions.
Dosage of serum estradiolThe serum levels of estradiol were measured by ELISA according to the manufacturer of the kit used (Estradiol ELISA Kit -Item No. 501890. Cayman Chemical’s ACE™, Ann Arbor, MI, USA).
ImmunohistochemistryAfter processing and embedding samples in paraffin, 4-mm histological sections were used for immunohistochemistry analysis. The sections were deparaffinized through a series of “washing” in xylene and alcohol. Endogenous peroxidase was quenched with 3% hydrogen peroxide for 10min at room temperature. Antibodies were used specific for macrophages CD68 (AbD Serotec, Germany - 1:100), being a marker of inflammatory response in tissue. A biotinylated anti-mouse secondary antibody (ImmPRESS Universal Reagent, Vector Laboratories, United States - 1:200) was used. Subsequently, we used the avidin-biotin complex. The color development occurred by adding the chromogen 3,3-diaminobenzidine (DAB, 3,3-diaminobenzidine, Easypath, Brazil) on the cuts by the reaction thereof with the avidin-biotin-peroxidase complex. The counterstaining was conducted by Harris hematoxylin washing. The number of CD68 positive cells was counted in 30 fields (measuring 0.087μm2 each) under light microscopy (Olympus BX51, Japan).15 All experiments were performed in triplicate.
Lung gene expression evaluation by RT-qPCR arrayThe gene expression of inflammatory markers was assessed by RT-qPCR array methodology. The mRNA of the lung tissue extracted by using TRIzol® Plus RNA Purification Kit (Thermo Scientific, São Paulo, Brazil) following the protocol supplied by the manufacturer. The cDNA obtained using SuperScript® III Reverse Transcriptase Kit was used in a customized SYBR Green qPCR reaction to determine expression of IL-1β, IL-6, and TNF-α genes (Qiagen-SABioscience, São Paulo, Brazil). Genes MGDC control (Mouse Genomic DNA Contamination), RTC (Reverse Transcription Control) and PPC (Positive PCR Control) were also included. The reaction was carried out using the StepOnePlus Real-Time PCR System (Thermo Scientific, São Paulo, Brazil). Cycling conditions were as follows: 50°C for 10min; 95°C for 10min; and 45 cycles of denaturation at 95°C for 15s, annealing at 60°C for 1min. The melting curve was evaluated at the end of the reaction to determine the specificity of the amplification. Analysis of gene expression data was performed using the 2−ΔΔCT method.17 GAPDH was used as an endogenous gene to evaluate the overall cDNA content.18 All experiments were performed in triplicate with three independent repetitions.
Statistical analysisStatistical analysis was performed using the GraphPad-Prism 6.0 program. The comparisons made in the different experiments were determined by individual variation or error variation (s2). To verify the distribution of data normality, the Shapiro-Wilk test was applied, which indicated that the sample did not follow a normal distribution and, therefore, the statistical analysis was continued with one-tailed Mann–Whitney non-parametric test. The results were expressed as mean±standard deviation of the mean (DPM). Statistically significant differences were considered when p-value<0.05 using a 95% confidence interval.
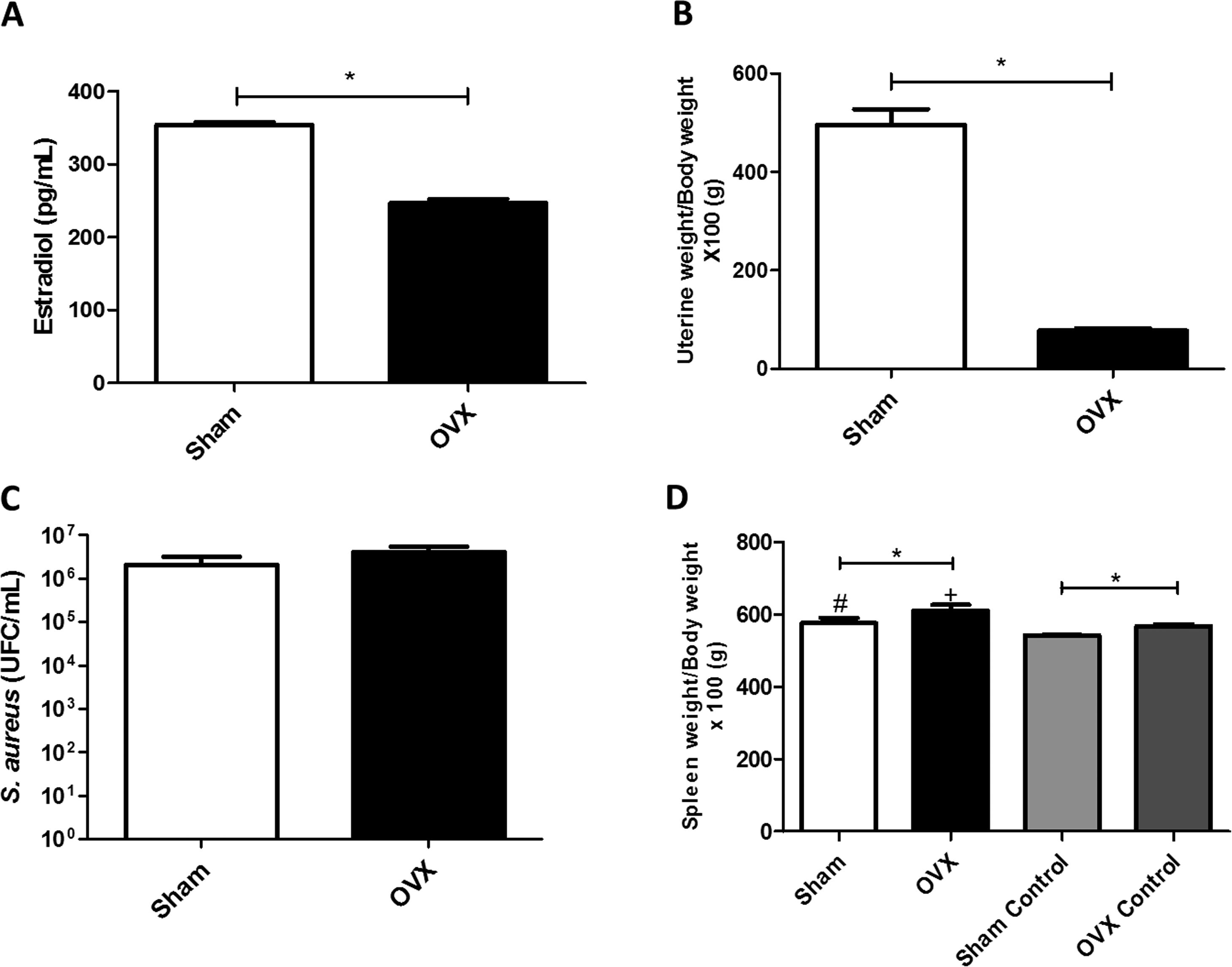
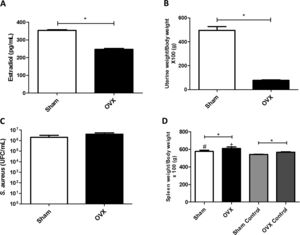
ResultsUterine index and dosage of serum estradiolTo evaluate the efficacy of ovariectomy, uterus index and dosage of serum estradiol were determined. Estrogen deficiency causes uterine atrophy, observed by a decrease in volume and weight of this organ. The OVX females of this study, therefore, had reduced levels of estradiol (Fig. 2A) and lower uterine weight (Fig. 2B) compared to sham females.
(A) Concentration of serum estradiol of female mice measured by ELISA (average value of all animals in the group). (B) Uterine weight-to-body weight of female mice subjected to sham surgery (Sham) or ovariectomized (OVX) (average value of all animals in the group). (C) Quantitation of S. aureus in blood of female mice subjected to or ovariectomized (OVX) inoculated with the ATCC 25923 strain of S. aureus measured by qPCR (average value of all animals in the group). UFC: Colony Forming Units. (D) Spleen weight-to-body weight of female mice subjected to sham surgery (sham) or ovariectomized (OVX) inoculated with the ATCC 25923 strain of S. aureus or saline (Sham Control/OVX Control) (average value of all animals in the group). Data are expressed as mean±SD. Statistical significance (p<0.05) is represented by the symbols (*difference among Sham and OVX groups; with the negative control group, #difference with Sham Control; + difference with OVX Control).
The infection process caused bacteremia (presence of bacteria in the blood). There was systemic presence of S. aureus in both sham and OVX females; however, there was no statistical difference in bacterial load between the groups (Fig. 2C).
Spleen indexIn this study, OVX females had a greater increase in spleen weight compared to sham females, even in the absence of infection. Also, infected females had an enlarged spleen compared to control females (Fig. 2D).
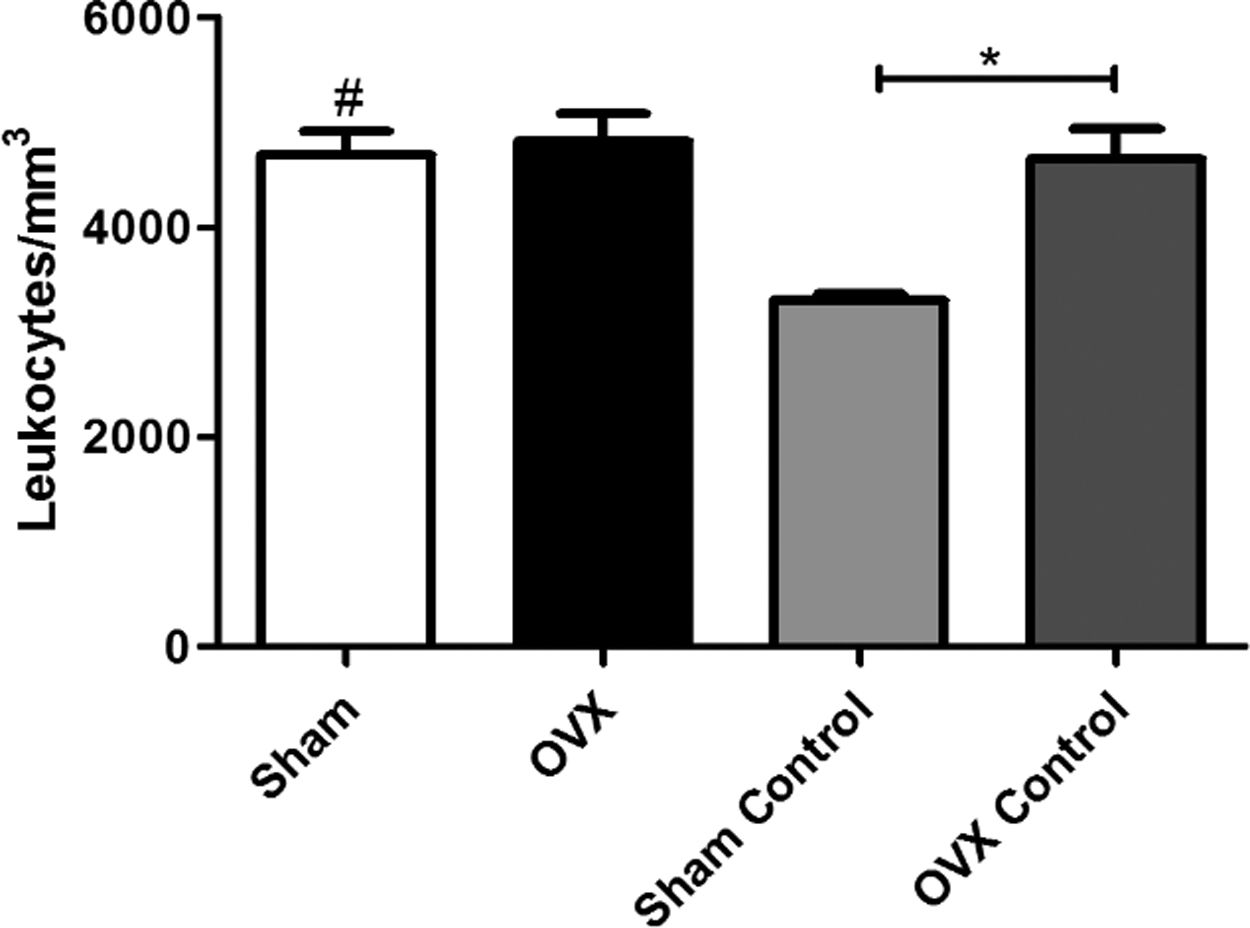
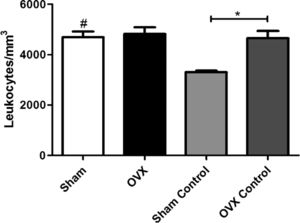
Total leukocyte countAn increase in the number of leukocytes in the sham group was observed compared to the sham control group; however, there was no difference between the OVX and OVX control group. The number of leukocytes in the OVX control group was significantly greater than the sham control group, despite the absence of infectious focus in these groups (Fig. 3).
Blood leukocyte counts of female mice sham surgery (Sham) or ovariectomized (OVX) inoculated with the ATCC 25923 strain of S. aureus or saline (Sham Control or OVX Control). Data are expressed as mean±SD. Statistical significance (P<0.05) is represented by the symbols (*difference among Sham and OVX groups. with the negative control group, #difference with Sham Control; + difference with OVX Control).
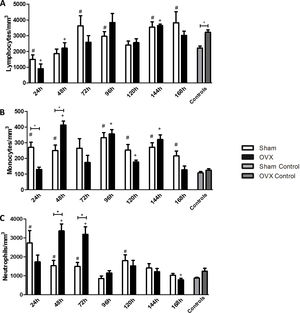
In the differential leukocyte count we took into account monocytes, neutrophils, and lymphocytes.
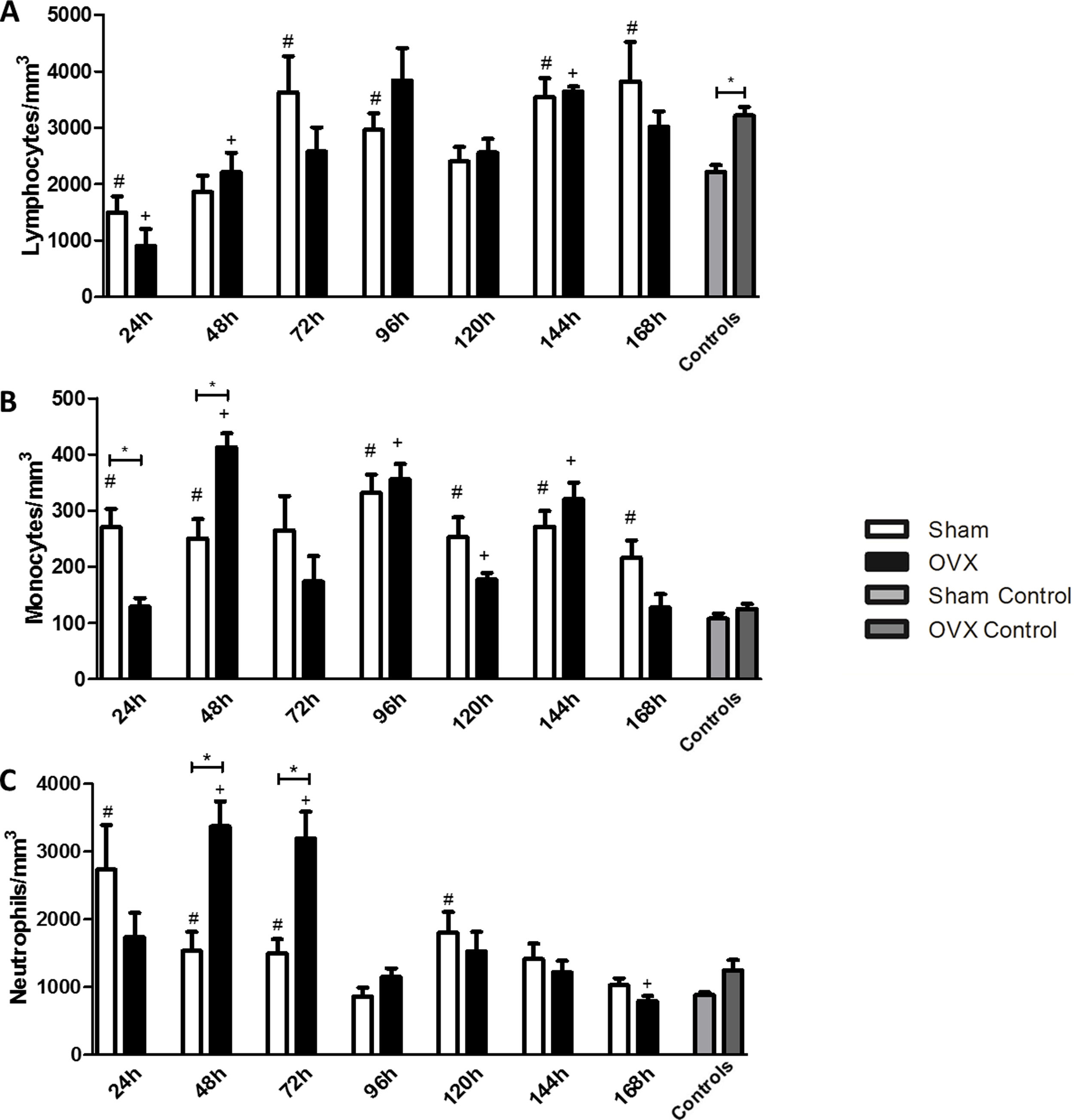
LymphocytesWe found that in the first 24h after infection, the infected groups had a lower number of lymphocytes compared to the controls. The lymphocyte reduction was observed in the first 24h in the sham group, while in OVX group this reduction was sustained until the 48h of infection compared to the control. An increase in the number of lymphocytes was observed at 72h of infection in the sham group, while such an increase was observed in the OVX group only after 144h of infection (Fig. 4A).
Blood lymphocyte (A), monocyte (B) and neutrophil (C) counts of female mice sham surgery (Sham) or ovariectomized (OVX) inoculated with the ATCC 25923 strain of S. aureus or saline (Sham Control or OVX Control). Data are expressed as mean±SD. Statistical significance (P<0.05) is represented by the symbols (*difference among Sham and OVX groups. with the negative control group, #difference with Sham Control; + difference with OVX Control).
The sham group had a monocyte increase in the first 24h after infection compared to the control. This increase in the monocyte response of the sham group remained at the time of 48, 96, 120, 144 and 168h. In the OVX group the increase occurred later, only at 48h, and remained in time of 96, 120 and 144h. A difference in the number of monocytes was observed between the sham and OVX group at 24 and 48h. At 24h, the sham group had an increased monocyte response compared to the OVX group, but at 48h, the OVX group showed the highest response (Fig. 4B).
NeutrophilsThe number of neutrophils increased significantly in the sham group in the first 24h of infection and remained until 72h with a new peak at 120h. However, in the OVX group, the number of neutrophils increased only at 48h and persisted until 72h of infection. Although the duration of neutrophil response in ovariectomized females was shorter, the response was exacerbated. Still, at 168h, in the OVX group, neutrophils decreased causing neutropenia (Fig. 4C).
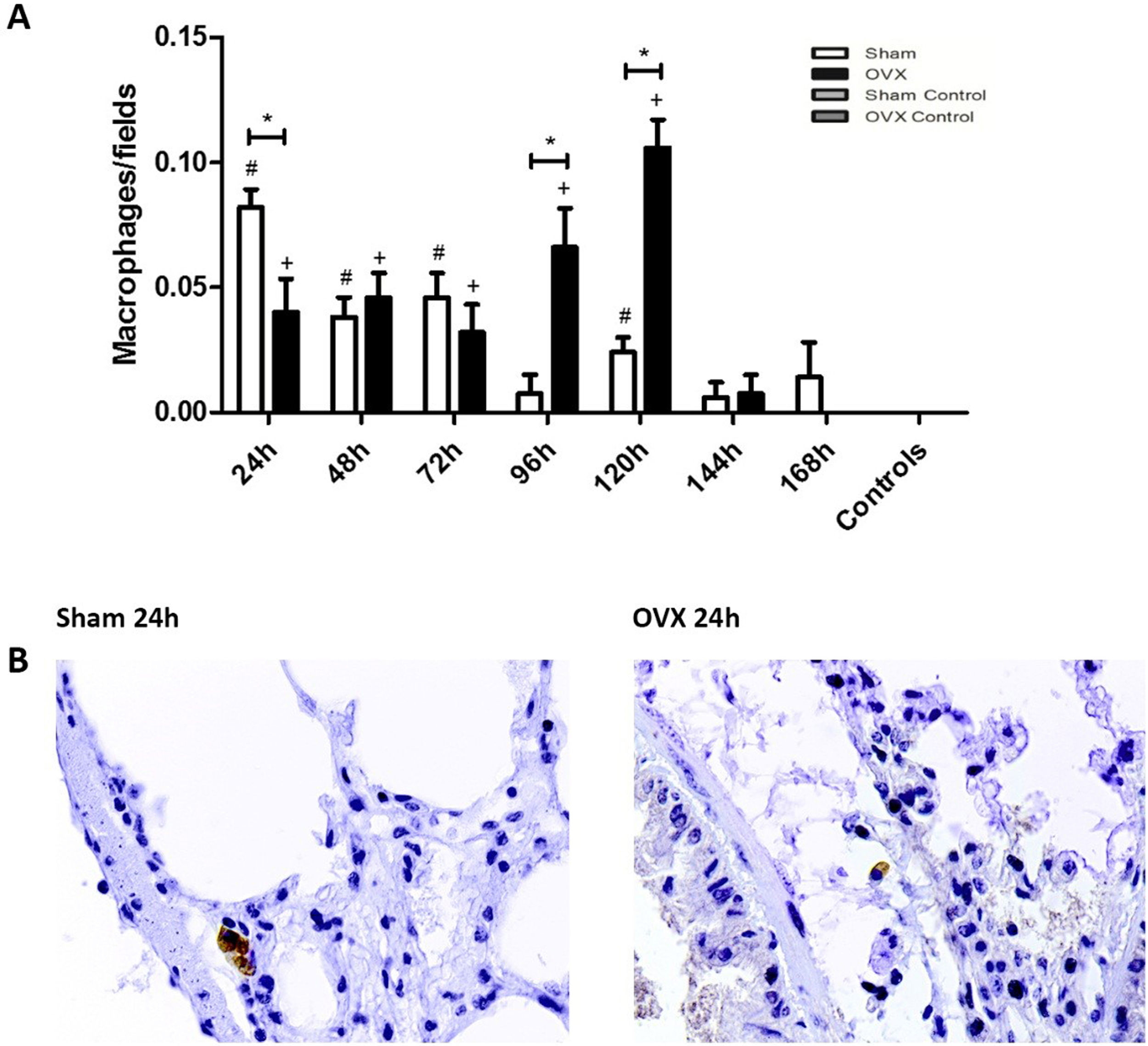
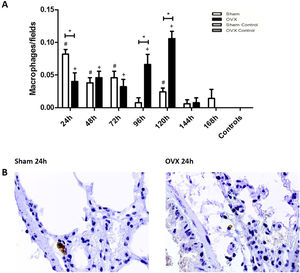
ImmunohistochemistryThe macrophage response in the lungs occurred more sharply in the shams than in the ovariectomized ones in the initial 24h of infection. However, at 96 and 120h, the number of macrophages of ovariectomized females was significantly higher (Fig. 5).
Immunolocalization of macrophages in lung tissue sections from female mice sham surgery (Sham) or ovariectomized (OVX) inoculated with the ATCC 25923 strain of S. aureus or saline (Sham Control or OVX Control). (A) Number of macrophages per field. Data expressed as mean±SEM. Statistical significance (P<0.05) is represented by the symbols (*difference among Sham and OVX groups, with the negative control group, #difference with Sham Control; + difference with OVX Control). (B) Representative photomicrographs of immunolocalization of macrophages (original magnification ×400).
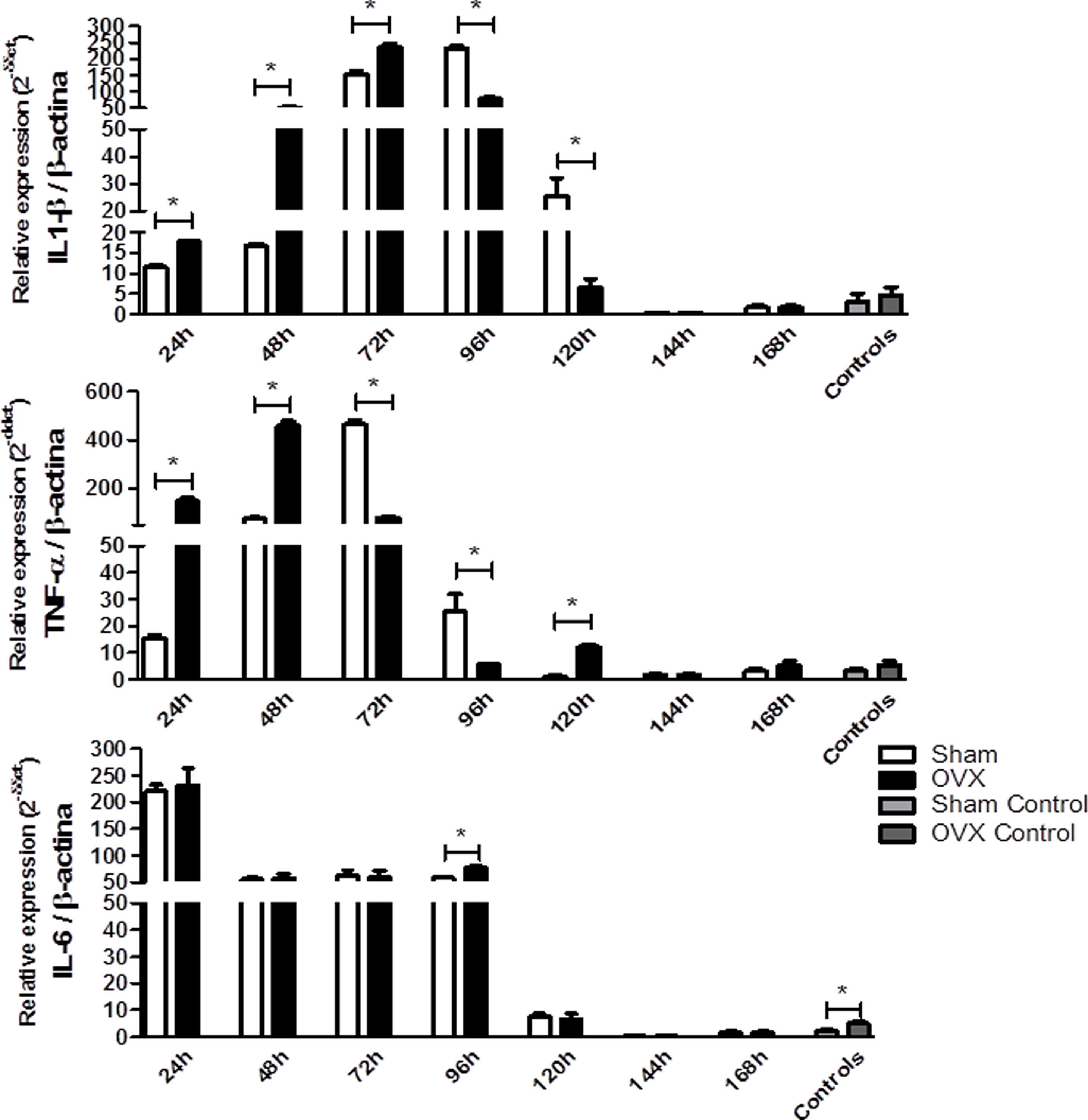
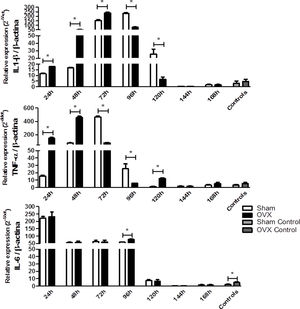
Because of the injuries observed in the lung tissue, we determined the relative gene expression of the cytokines IL-6, IL-1β and TNF-α by qPCR. The IL-1β and TNF-α responses were more rapid and robust in OVX group. The OVX group had a higher relative expression of IL-1β and TNF-α genes in the first 72h and 48h of infection compared to the sham group, respectively. However, after this period there was a change in this profile, so the sham group showed higher gene expression. Regarding IL-6 expression, after 96h of infection we found that the OVX group had increased expression of this gene compared to the sham group. Interestingly, we found that ovariectomized females had higher IL-6 gene expression in the lung compared to sham females, even in the absence of infection (Fig. 6).
Relative gene expression of the cytokines IL-1β (A), TNF-α (B) and IL-6 (C) per time of infection in the lungs of female mice sham surgery (Sham) or ovariectomized (OVX) inoculated with the ATCC 25923 strain of S. aureus or saline (Sham Control or OVX Control) measured by qPCR. Data are expressed as mean±SD. * P<0.05; # P<0.05 vs Sham Control; + P<0.05 vs OVX Control.
Experimental and clinical studies indicate sex-specific differences in infectious diseases9–11; in part, these differences are attributed to sex hormones.20 However, there is little information regarding the role of ovarian hormones in infections by Gram-positive bacteria, such as S. aureus. Ovariectomy significantly reduces the level of ovarian hormones, and the success of this surgery can be confirmed by uterine atrophy21 and by reduction of serum estradiol levels, as was seen in all ovariectomized females compared to shams of this study. In the present study, there was no difference in bacterial load in blood between shams and ovariectomized females. Hanses et al.22 found no significant differences in relation to sex in survivors to S. aureus bacteremia. However, McGowan et al.23 reported a significantly higher incidence of bacteremia in men compared to women. In an experimental model, Erikoglu et al.24 found that Sprague-Dawley female rats with sepsis had lower endotoxemia compared to male rats, and this process could be related to the presence of female sex hormones.
Our results showed that, even in the absence of infection, the ovariectomized females had a larger spleen than sham females. The spleen has an important lymphatic immune function due to the production of immune cells, and infectious diseases can cause an enlarged spleen. This splenomegaly suggests that the immune responses mounted in the spleen were more exacerbated in ovariectomized than in shams, which is probably related to the levels of sex steroids. Youssef and Stashenko25 observed a significant enlargement of the spleen in female ovariectomized mice that received anti-IL-1α/β antibodies, and splenomegaly was almost completely reversed by supplementation with E2. Cunningham et al.26 observed that NZM2410 ovariectomized mice showed a tendency towards larger spleens, although they did not observe a statistically significant difference. In a study carried out with Wistar rats, ovariectomized females showed a hyperplasia of the white pulp of the spleen in relation to the non-ovariectomized group. This result occurred due to the increase in the number of lymphoid follicles compared to the control group, but the increase in white pulp was accompanied by a reduction in the percentage of red pulp, which meant that the spleen weight/body weight ratio in that study was not significantly higher compared to the control group.27 However, Dai et al.28 observed that, compared to placebo-treated NZB/WF1 mice, mice exposed to E2 showed a significant increase in absolute spleen weight and splenic index.
In general, there was no difference in the number of total leucocyte counts between shams and ovariectomized infected females. As expected, an increase in the number of leukocyte counts was observed in the sham-infected group compared to the sham control group. However, no difference was observed between the OVX infected and OVX control group. Also, we found increased leukocytes in the OVX control group compared to the sham control group, leading us to believe that lack of ovarian hormones may cause a significant increase in leukocyte counts even in the absence of infection. By analyzing the white blood cell differential counts, only the lymphocytes were increased in the OVX control group. Therefore, these findings suggest that the absence of ovarian hormones alone may be able to cause a significant increase in circulating lymphocytes. In a study by Perisic et al.29 evaluating the role of ovarian hormones on the homeostasis of the T lymphocytes, ovariectomy increased the number of CD4+ and CD8+ lymphocytes in blood and spleen due to a decreased lymphocyte apoptosis rate in the periphery and spleen. de Oliveira et al.13 observed that ovary removal could also increase the frequency of Treg lymphocytes in the spleen and lymph nodes and that hormone replacement therapy with estrogen and progesterone overrode the ability to induce the expansion of Treg of the ovariectomy.
In our model, it was observed that in the initial hours of infection, lymphocyte counts were significantly reduced in infected females compared to controls. In shams, this reduction was seen after 24h of infection, while this reduction in OVX females continued up to 48h of infection. Patients with sepsis are usually lymphopenic; in a study by Hotchkiss et al.30 a stratified analysis of spleen autopsy tissue samples showed that there was selective depletion of B lymphocytes and CD4+. This process and its functional consequences are part of a general state of immunosuppression, characterized by hyporesponsiveness of T lymphocytes and anergy, which occur in most septic patients and is considered a counter response, to balance (and sometimes overlapping) the initial pro-inflammatory state.31
Monocytes increased in the first 24h of infection in the sham group that was significantly higher compared to the OVX group, which remained with absolute numbers of monocytes similar to the control group. In turn, in OVX females monocytes counts increased only after 48h of infection. Indeed, the number and activity of innate immune cells, including monocytes and macrophages, are generally higher in women than in men, which may be closely related to levels of ovarian hormones, especially estrogen.32 However, evidence of the link between estrogen levels and circulating monocytes are contradictory, since during menopause and in males there is an increase in monocyte counts in blood compared to women in the follicular phase.33 Studies show that post-menopausal women treated with hormone replacement therapy (HRT), estrogen agonists or even isoflavones, have low serum levels of ICAM-1, E-selectin and VCAM-1 compared to untreated postmenopausal women.34–36
Neutrophils showed a similar behavior to that seen with monocytes. We observed an increased number of neutrophils in the first 24h of infection in the sham group, and this profile remained for a few days, reaching homeostasis levels after 144h of infection. In OVX females, we observed an increase in neutrophil counts only after 48h of infection and that response continued only for another 24h. However, in the days when this increase was observed, the neutrophil response of the OVX group was significantly more pronounced than in the sham group. It has been shown that progesterone enhances neutrophil chemotactic activity,37 while E2 reduces such chemotaxis.38 Molloy et al.39 found a significant delay in spontaneous rate of apoptosis of neutrophils in women in reproductive age compared with men in the same age. Moreover, use of estrogen and progesterone antagonists reversed the apoptotic delay. The findings of our study indicate that the presence of female sex hormones implies a faster, longer and less accentuated neutrophil response, which is probably less harmful in infection induced by S. aureus. Nonetheless, in ovariectomized females this response occurs late, and neutropenia observed after 168h of infection suggests a greater chance of reinfection and a likely indication of immunoparalysis.
In the present study, the histological evaluation of the lungs showed sharp hyperemia, presence of bleeding points, and enlargement of alveolar septa. When the number of macrophages in the lung tissue was analyzed by time of infection, the sham group presented a more marked response compared to ovariectomized females in the first 24h of infection, as observed in blood. In general, data in the literature indicates that female sex hormones are related to less lung damage. Septic female and male Sprague-Dawley rats treated with estrogen and progesterone showed less damage to lung tissue than untreated or testosterone-treated rats.24 Caruso et al.40 demonstrated that female sex hormones protect against hemorrhagic trauma-induced lung injury followed by shock due to decreased capillary permeability to leukocytes.
The triggering stimuli of systemic inflammation are often bacterial components, which induce secretion of pro-inflammatory cytokines, which are critical factors in the acute phase of inflammatory response.41 In this study, it was observed that ovariectomized females showed higher relative expression of IL-6 in the lungs, even in the absence of infection. Menopause may cause an increase in IL-6 levels in serum42,43 and this increase is diminished with HRT, more precisely due to the estrogen.44,45 Furthermore, elevated IL-6 plasma levels were seen in seriously injured men compared to women in the same condition46 and E2 treatment following trauma-hemorrhage decreased plasma IL-6 (and TNF-α).47 In an air pouch model, Stubelius et al.48 observed that OVX mice showed a significant increase in IL-6 levels compared to female shams, and these levels were reduced after treatment with E2. Naugler et al.49 observed that estrogen inhibited the secretion of IL-6 by Kupffer cells. In addition, researchers observed that E2 administration in OVX rats reduced the expression of IL-6 in the liver compared to untreated OVX rats.
A higher relative expression of the IL-1β gene in the lung tissue at the beginning of infection was observed in OVX compared to the sham group. These findings suggest that the induction of inflammatory response in early infection is more robust in ovariectomized compared to sham females. Schaefer et al.50 demonstrated that estradiol inhibits IL-1β response by binding to estrogen receptors because the addition of a receptor antagonist abrogated this effect. In homeostasis situations, estrogen levels inhibit the production of this cytokine. The effects of estradiol in the production of IL-1β are still contradictory. In women, an increase in plasma levels of progesterone and 17β-estradiol were associated with an increase in the percentage of IL-1β (and TNF-α) by peripheral monocytes after stimulation with endotoxin in vitro.51 Lefevre et al.52 found no differences in IL-1β levels in blood samples of men and women after incubation with LPS for 24h.
The OVX group had higher TNF-α gene expression in the lung tissue at the beginning of infection compared to the sham group. This result in particular suggests, once more, that induction of an inflammatory response in early infection is greater in ovariectomized compared to sham females. TNF-α secretion has been described as biphasic53 and studies show different estrogen effects of this cytokine. Du et al.54 found significantly lower TNF-α gene expression in septic lungs of female rats compared to males two hours after LPS challenge, and Frink et al.47 observed that E2 treatment following trauma-hemorrhage decreased plasma TNF-α levels, which was associated with less lung neutrophil infiltration. Cioffi et al.42 observed that, compared to fertile women, postmenopausal women have lower concentrations of TNF-α.
The results of this study demonstrated that ovariectomy and therefore the lack of ovarian hormones cause an adverse immune response compared to females with intact ovaries even in the absence of infection. It was observed that the presence of estrogen was immunoprotective because it was related to a faster response of the immune cells. Hormonal fluctuations occur throughout the period of the ovarian cycle and during pregnancy for women. In addition, due to increased life expectancy, menopause and their effects on health are a key concern. In this context, the experimental model developed in this work contributes to the study of the influence of ovarian hormones in response to a globally recurrent disease such as infection induced by S. aureus.
Funding informationThis study was supported by Fundação de Amparo à Pesquisa do Estado da Bahia (BOL0824/2011), Programa de apoio a pesquisadores emergentes da UFBA (PRODOC 02/2011) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Code 001).
Ethical statementAll experiments were conducted in accordance with internationally accepted principles for the use and care of laboratory animals as set out in the European Community guidelines (EEC Directive 1986/86/609) and executed after approval by the Animal Ethics Committee (AEC) of the IMS/UFBA, under protocol nº 03/2013.
Conflicts of interestThe authors declare that there are no conflicts of interest.
We thank Aricelma P. França for invaluable technical assistance and AcademicEnglishSolutions.com for reviewing the English.