This retrospective study was aimed to explore the epidemiological and clinical profiles of Mycoplasma pneumoniae infection in neonates.
MethodsFrom 2011 to 2014, 1322 hospitalized neonates with lower respiratory tract infections were screened for Mycoplasma pneumoniae by detection of Mycoplasma pneumoniae antibodies using Serion ELISA classic Mycoplasma pneumoniae kits.
ResultsMycoplasma pneumoniae was identified in 89 (6.7%) patients. The age ranged from 1 day to 28 days with a median of 22 days. The male to female ratio was 1.15:1. Mycoplasma pneumoniae infection peaked in spring (from March through May) and winter (from December through February). Compared with non-Mycoplasma pneumoniae infected neonates, those with Mycoplasma pneumoniae infection were older, presented fever more frequently, and had less tachypnea.
ConclusionsMycoplasma pneumoniae could be an important etiologic agent for respiratory tract infection in neonates. In neonates Mycoplasma pneumoniae infection was usually associated with older age, presence of fever, and less tachypnea. Mycoplasma pneumoniae infection in neonates tends to be a mild process.
Mycoplasma pneumoniae (MP) is an important cause of community-acquired pneumonia in children.1,2 MP causes respiratory tract infection in all age groups.3 Nonetheless, the main burden of infection is typically found in school-age children. An increasing incidence of MP infection and disease in children and infants under five years is being reported.4–7 However, the relevance of MP as an etiological agent of neonatal lower respiratory tract infections (LRTIs) remains unclear.
To the best of our knowledge, MP as an etiological agent of neonatal LRTIs has occasionally been reported,8,9 but no studies have been conducted to elucidate the role of MP in neonatal LRTIs in China and other regions throughout the world. Thus it is important to delineate the extent of involvement of MP in neonatal LRTIs.
Toward that end, we investigated the occurrence of MP infection in 109 hospitalized neonates with LRTIs from 2011 to 2014 and further analyzed the incidence, clinical, and laboratory data of these cases.
Materials and methodsPatientsA total of 1322 neonates hospitalized with LRTIs in Children's Hospital of Soochow University, China, from Jan 2011 to Dec 2014 were selected for this observational retrospective study. To be included in this study, patients had to be aged less 29 days and be hospitalized for LRTIs during the study period. LRTI was defined as the presence of at least three of the following signs and symptoms: cough, tachypnea, chest retractions, abnormal auscultatory findings (wheezing or crackles), and radiologic evidence indicative of lower respiratory tract infection. The exclusion criteria were: neonates with a vertical infection (i.e., an infection transmitted from mother to child) or infection acquired during delivery. The study was approved by the Ethics Committee of Children's Hospital of Soochow University.
Data collectionPatient demographic data, laboratory and radiographic reports were collected and reviewed. Laboratory data including peripheral white blood cell count, neutrophil and lymphocyte proportions, C-reactive protein (CRP), serology for MP, and nasopharyngeal specimens for viral testing were recorded and further analyzed.
Serology for MPSpecific IgM and IgG antibodies against MP were detected in serum samples of patients in the acute phase of MP (on admission) and convalescent phase (on discharge), using a commercial ELISA kit (Serion ELISA classic MP IgG/IgM, Institute Virion/Serion, Würzburg, Germany) according to the manufacturer's instructions. The presence of IgM in the convalescent phase was used as criteria of current MP infection.
Nasopharyngeal specimen collectionNasopharyngeal secretions were collected from each study participant within 24h after admission by a lab technician as previously described. Briefly, an aseptic plastic sputum catheter was inserted into the nostril to a depth of about 7–8cm until reaching the pharynx. Approximately 2mL of nasopharyngeal secretions was collected by applying negative pressure. The sample was mixed with 4–8mL PBS, and centrifuged for 10min at 300–500rpm. The supernatant was discarded and the pellet was mixed with 4–8mL PBS and centrifuged for an additional 10min. The specimens were centrifuged and were stored at −80°C until tested.
Detection of virusesAll nasopharyngeal swabs were tested by immunofluorescence for antigen detection of seven common viruses, including respiratory syncytial virus (RSV), adenovirus (ADV), influenza viruses (IV) A and B, and parainfluenza viruses (PIV) 1, 2 and 3.
Statistical analysisWe used n (%) for categorical variables and median (range) for continuous variables with non-normal distribution or mean (SD) for those with normal distribution. We assessed differences in categorical variables with the χ2 test. We calculated 95% CI for differences in medians with an exact test. SPSS (version 17.0) software was used for all statistical analysis. Multivariate logistic regression analysis was used to analyze variables associated with detection of MP.
ResultsPatient demographicsA total of 1322 neonates hospitalized with LRTIs were included in this study. Of those, MP was identified in 89 (6.7%) patients, with RSV in 255 (19.3%) cases, 59 (4.7%) with IV-A, 13 (1.0%) with PIV-3, 9 (0.7%) with IV-B, and 8 (0.6%) with ADV. Multi-viruses were identified in 36 (2.7%) patients. Among the 89 patients with MP infection, 16 patients (17.8%) were co-infected with RSV, and one (1.1%) was co-infected with influenza A.
Age ranged from 1 day to 28 days with a median of 22 days. The male to female ratio was 1.15:1. The birth weight ranged from 2150 to 4000g with a median of 3450g. Gestational age ranged from 33 to 41 weeks with a median of 37 weeks.
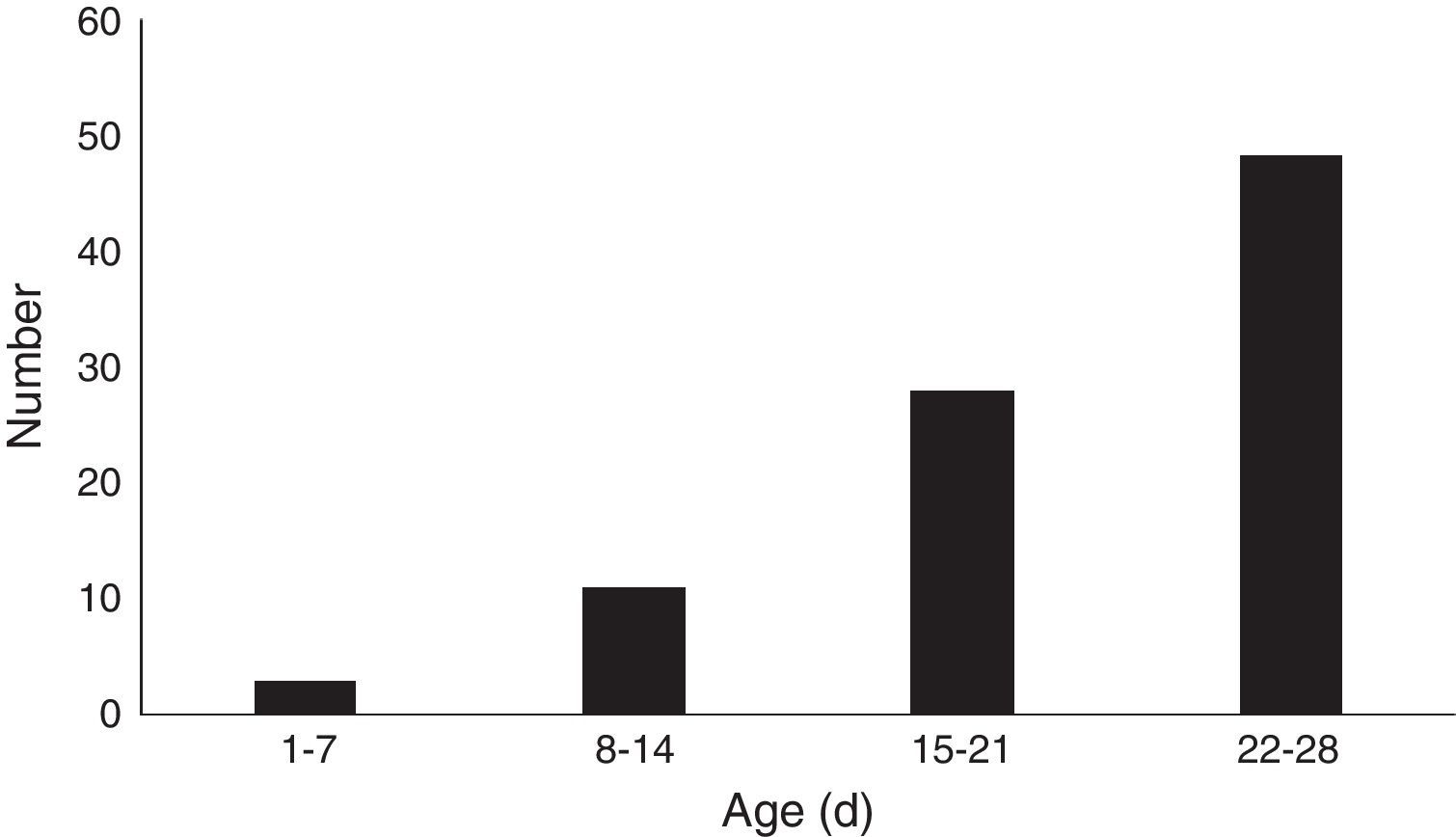
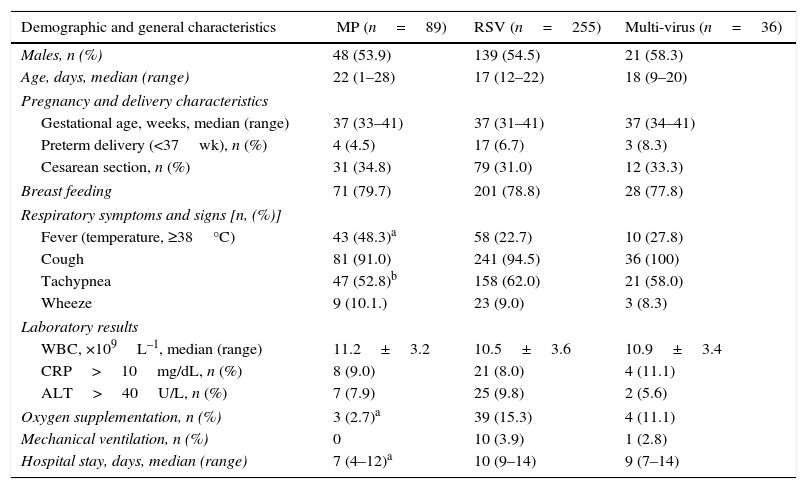
Epidemiology of MP infectionThe age distribution of MP infection in neonates is shown in Fig. 1. Three (3.4%) cases were aged 1–7 days, 11 (12.4%) 8–14 days, 28 (31.5%) 15–21 days, and 47 (52.8%) were aged 22–28 days. Thus, the incidence of MP infection in neonates increases with age.
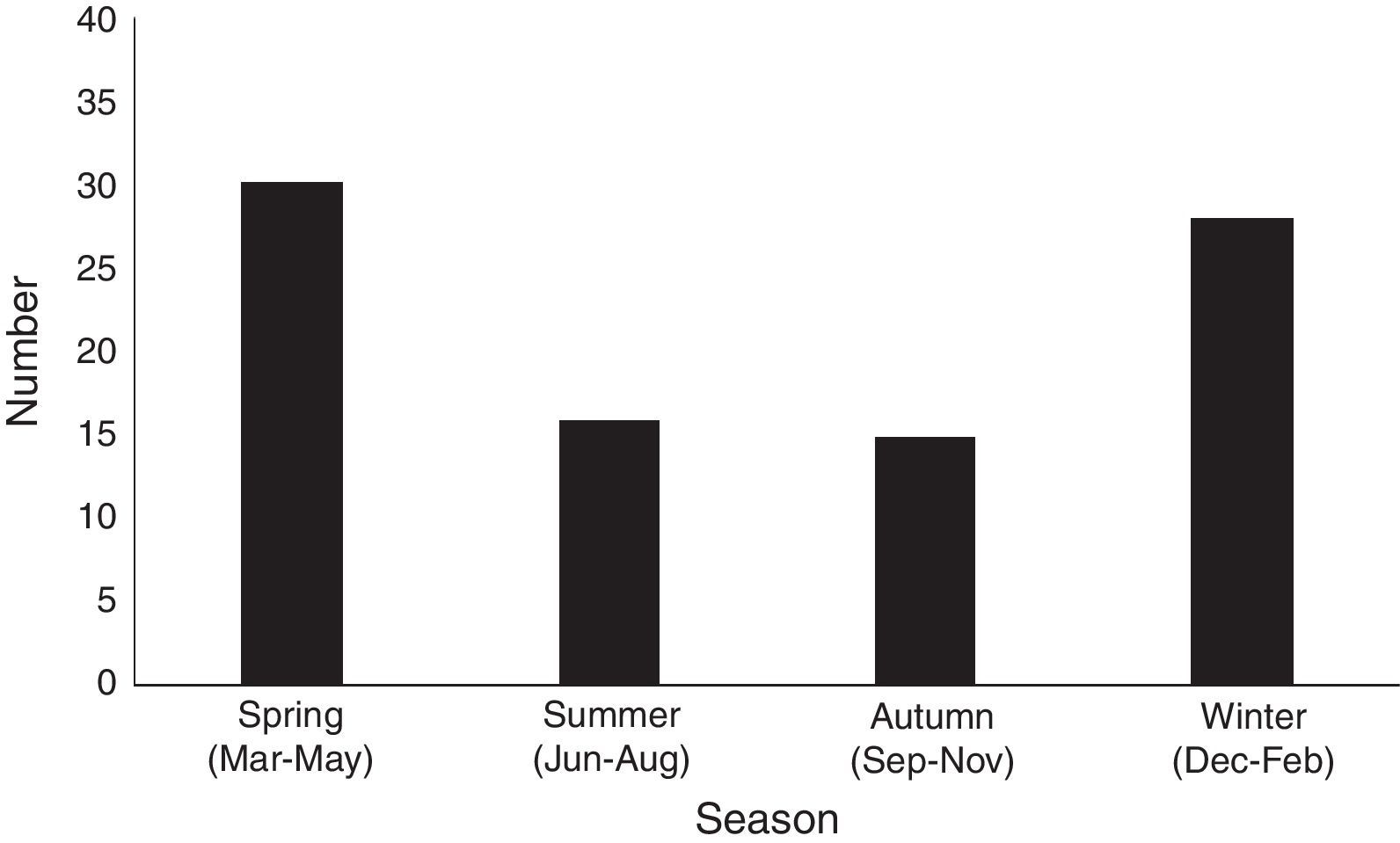
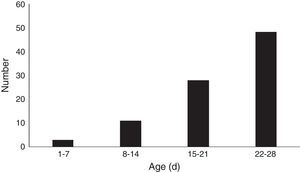
The monthly distribution of MP infection in neonates is shown in Fig. 2. MP infection peaked in spring (from March through May) and winter (from December through February), which involved 30 cases (33.7%) in spring, and 28 (31.5%) cases in winter, respectively, compared with 16 cases (18.0%) in summer (June to August), and 15 (16.9%) in autumn (from September to November). The prevalence of MP infection was higher during winter and spring, and lower in summer and autumn.
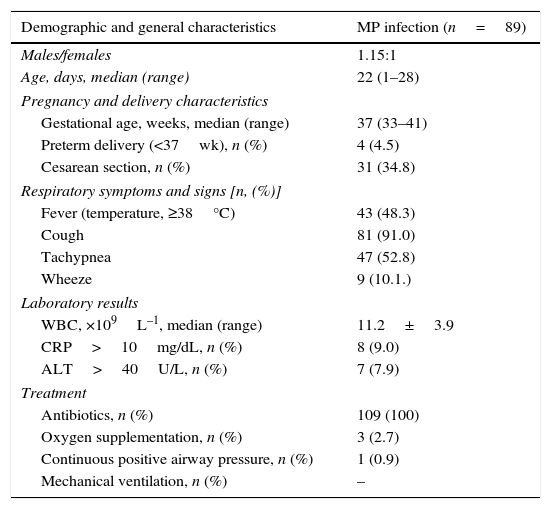
Clinical features, laboratory and radiographic findingsThe main clinical features of the patients with MP infection are shown in Table 1. Eighty-one (91.0%) patients had cough. Dyspnea or tachypnea was observed in 47 (52.8%) patients, wheezing in 9 (10.1%), and fever (temperature, >38°C) in 43 (48.3%) patients. Mean WBC counts were 11.2±3.2×109L–1; CRP were >10mg/mL in eight (9.0%) patients. Liver enzyme, alanine aminotransferase level was above the upper limit of normality in seven (7.9%) patients.
Demographic and general characteristics of neonates with Mycoplasma pneumoniae (MP) infection.
| Demographic and general characteristics | MP infection (n=89) |
|---|---|
| Males/females | 1.15:1 |
| Age, days, median (range) | 22 (1–28) |
| Pregnancy and delivery characteristics | |
| Gestational age, weeks, median (range) | 37 (33–41) |
| Preterm delivery (<37wk), n (%) | 4 (4.5) |
| Cesarean section, n (%) | 31 (34.8) |
| Respiratory symptoms and signs [n, (%)] | |
| Fever (temperature, ≥38°C) | 43 (48.3) |
| Cough | 81 (91.0) |
| Tachypnea | 47 (52.8) |
| Wheeze | 9 (10.1.) |
| Laboratory results | |
| WBC, ×109L–1, median (range) | 11.2±3.9 |
| CRP>10mg/dL, n (%) | 8 (9.0) |
| ALT>40U/L, n (%) | 7 (7.9) |
| Treatment | |
| Antibiotics, n (%) | 109 (100) |
| Oxygen supplementation, n (%) | 3 (2.7) |
| Continuous positive airway pressure, n (%) | 1 (0.9) |
| Mechanical ventilation, n (%) | – |
Chest radiographs of all patients were available for review. Peribronchial infiltration and perihilar infiltration were observed in 81 (91.0%) children. Homogenous consolidation was present in seven (7.9%) patients. None of the patients presented with pleural effusion.
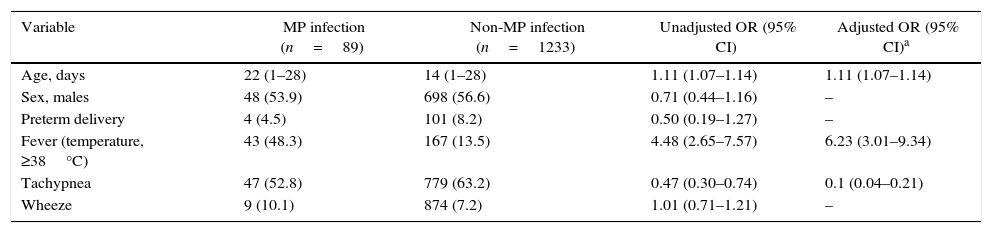
Variables associated with MP infectionDemographic and clinical characteristics from Table 1 were compared between neonates with MP infection (n=89) and those with non-MP infection (n=1233). From this univariate analysis, three predictor variables, namelyage, fever (temperature≥38°C) and tachypnea were evaluated in a logistic regression model: compared to non-MP cases MP cases were older (median of 22 days vs 14 days), had more fever (48.3% vs 13.5%), and less tachypnea (52.8% vs 63.2%) (see Table 2 for statistics).
Risk of Mycoplasma pneumoniae (MP) infection per selected variable.
| Variable | MP infection (n=89) | Non-MP infection (n=1233) | Unadjusted OR (95% CI) | Adjusted OR (95% CI)a |
|---|---|---|---|---|
| Age, days | 22 (1–28) | 14 (1–28) | 1.11 (1.07–1.14) | 1.11 (1.07–1.14) |
| Sex, males | 48 (53.9) | 698 (56.6) | 0.71 (0.44–1.16) | – |
| Preterm delivery | 4 (4.5) | 101 (8.2) | 0.50 (0.19–1.27) | – |
| Fever (temperature, ≥38°C) | 43 (48.3) | 167 (13.5) | 4.48 (2.65–7.57) | 6.23 (3.01–9.34) |
| Tachypnea | 47 (52.8) | 779 (63.2) | 0.47 (0.30–0.74) | 0.1 (0.04–0.21) |
| Wheeze | 9 (10.1) | 874 (7.2) | 1.01 (0.71–1.21) | – |
Data are presented as median (range) or n (%).
CI, confidence interval; OR, odds ratio.
Overall, three (3.4%) out of 89 patients with MP infection required supplemental oxygen. None required mechanical ventilation. The median length of hospital stay was seven (4–12) days. Duration of follow-up was one month for 83 children. None of the patients had residual pulmonary disease.
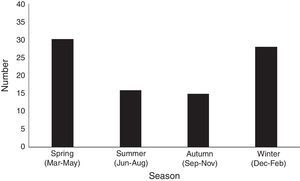
Comparison of clinical characteristics of patients with MP, RSV, and multi-virus infectionThe clinical characteristics of MP, RSV, and multi-viruses infection are shown in Table 3. The median age was 22 days for MP patients, 17 days for RSV patients, and 18 days for multi-viruses patients. Fever was more common in children with MP than either RSV or multi-viruses infection (both p<0.01). Tachypnea and oxygen supplementation were more often present in children with RSV than with MP infection (both p<0.01). The median length of hospital stay was longer in children with RSV and multi-viruses infection than for those with MP infection (both p<0.01).
Comparison of clinical characteristics of patients with MP, RSV and multi-virus infection.
| Demographic and general characteristics | MP (n=89) | RSV (n=255) | Multi-virus (n=36) |
|---|---|---|---|
| Males, n (%) | 48 (53.9) | 139 (54.5) | 21 (58.3) |
| Age, days, median (range) | 22 (1–28) | 17 (12–22) | 18 (9–20) |
| Pregnancy and delivery characteristics | |||
| Gestational age, weeks, median (range) | 37 (33–41) | 37 (31–41) | 37 (34–41) |
| Preterm delivery (<37wk), n (%) | 4 (4.5) | 17 (6.7) | 3 (8.3) |
| Cesarean section, n (%) | 31 (34.8) | 79 (31.0) | 12 (33.3) |
| Breast feeding | 71 (79.7) | 201 (78.8) | 28 (77.8) |
| Respiratory symptoms and signs [n, (%)] | |||
| Fever (temperature, ≥38°C) | 43 (48.3)a | 58 (22.7) | 10 (27.8) |
| Cough | 81 (91.0) | 241 (94.5) | 36 (100) |
| Tachypnea | 47 (52.8)b | 158 (62.0) | 21 (58.0) |
| Wheeze | 9 (10.1.) | 23 (9.0) | 3 (8.3) |
| Laboratory results | |||
| WBC, ×109L–1, median (range) | 11.2±3.2 | 10.5±3.6 | 10.9±3.4 |
| CRP>10mg/dL, n (%) | 8 (9.0) | 21 (8.0) | 4 (11.1) |
| ALT>40U/L, n (%) | 7 (7.9) | 25 (9.8) | 2 (5.6) |
| Oxygen supplementation, n (%) | 3 (2.7)a | 39 (15.3) | 4 (11.1) |
| Mechanical ventilation, n (%) | 0 | 10 (3.9) | 1 (2.8) |
| Hospital stay, days, median (range) | 7 (4–12)a | 10 (9–14) | 9 (7–14) |
MP, Mycoplasma pneumoniae; RSV, respiratory syncytial virus; WBC, white blood cell counts; CRP, C-reactive protein; ALT, alanine transaminase.
Respiratory infections are the most common cause of neonatal morbidity and mortality, despite many advances in neonatal intensive care. MP is often the etiological agent of respiratory infections in children older than five years and in adolescents.10 In recent years, the role of MP in children and infants under five years has been reported.5–7,11 However, no large sample studies have focused on neonatal MP infection. This retrospective study describes the epidemiology and clinical features of MP in neonates during four consecutive years. In that period, 8.2% of the neonates were found to be infected with MP. Previous studies12 have found that 21.6% of two-year old patients had positive throat swabs for MP. Wang et al. also found that bronchiolitis due to MP was mainly seen in infants aged 6 months to one year with a detection rate of 23.9%. In our study, the incidence of MP infection was lower than that shown in previous studies and concentrated in children less than two years.
During the early phase of MP infection, PCR can be a useful diagnosis tool in young or immunocompromised patients who may not be able to mount an adequate immune response to MP.13–15 However, a recent study indicated that the presence of MP in the upper respiratory tract is common in asymptomatic children.16 Realtime PCR therefore does not represent an unambiguous method for the diagnosis of symptomatic M. pneumoniae infections.16,17 Accordingly, in our study we used MP-IgM, instead of PCR, to diagnose MP infection. IgM is usually produced one to two weeks after the initial MP infection.2 The presence of IgM in the convalescent phase was a criterium for current MP infection. Therefore, we believe that our definition for MP infection was better surrogate marker for an existent MP infection in neonates with LRTI.
MP infections can occur endemically throughout the year or in epidemics. Epidemics of MP infection were common during the winter and spring in this study. However, previous studies showed that MP infection in children peaks in May through July (summer) and in August through October (autumn).11,18 Thus, the outbreaks of MP infection may differ in countries with different climates.
The clinical presentation of MP infection was compared with that of non-MP infection. MP infection was associated with older age and fever was more common compared with non-MP infection. However, tachypnea was less common in MP infection. Our findings are in line with our previous study which described the clinical profile of MP infection in children with bronchiolitis.4 Wang et al.4 found that patients with MP bronchiolitis were older and fever was more common, but tachypnea was less common in patients with MP bronchiolitis compared with RSV bronchiolitis. However, in the study by He et al.,3 tachypnea and fever were not significantly associated with respiratory infection due to MP. This difference may probably be explained by the different age range of the enrolled patients. The study reported by He et al. enrolled children of all age groups. The clinical profile of MP may differ with different age groups.
Non-resolving pneumonia caused by MP was reported in a neonate.9 However, in our study MP infection was generally a mild process in all neonates. MP single infection cases neither needed oxygen therapy nor required mechanical ventilation.
Our study has some limitations. First, our cases were identified retrospectively with data limited to that available in the medical records. The use of retrospective data also made it difficult to accurately assess the role of MP infection in neonates with LRTI. A second limitation was the absence of asymptomatic control patients.
ConclusionOur results suggest that MP might be an important etiologic agent for lower respiratory tract infection in neonates. MP infection in neonates was usually associated with older age, fever, and less tachypnea.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by theScience and Technology Program of Suzhou (SYS201435);Science and Technology Projects for the Youth of Suzhou (KJXW2015013); and the Science and Technology Program of Suzhou Health Bureau (lczx201409).