Since the advent of highly active antiretroviral therapy in 1996, we have seen dramatic changes in morbi-mortality rates from human immunodeficiency virus-positive patients. If on the one hand, the immunologic preservation-associated with the use of current antiretroviral therapy markedly diminishes the incidence of opportunistic infections, on the other hand it extended life expectancy of human immunodeficiency virus-infected individuals similarly to the general population. However, the management of critically ill human immunodeficiency virus-infected patients remains challenging and troublesome for practicing clinician. Sepsis – a complex systemic inflammatory syndrome in response to infection – is the second leading cause of intensive care unit admission in both human immunodeficiency virus-infected and uninfected populations. Recent data have emerged describing a substantial burden of sepsis in the infected population, in addition, to a much poorer prognosis in this group. Many factors contribute to this outcome, including specific etiologies, patterns of inflammation, underlying immune dysregulation related to chronic human immunodeficiency virus infection and delays in prompt diagnosis and treatment. This brief review explores the impact of sepsis in the context of human immunodeficiency virus infection, and proposes future directions for better management and prevention of human immunodeficiency virus-associated sepsis.
Although access to highly active antiretroviral therapy (HAART) has prolonged survival and improved life quality,1,2 human immunodeficiency virus (HIV)-infected patients with severe immunosuppression or comorbidities may develop complications that require critical care support in intensive care unit (ICU).
Causes of admissions to ICU in HIV-infected individuals have shifted from the traditional AIDS-related illnesses to non-AIDS-related diagnoses. Nevertheless, opportunistic infections still represent 30%–40% of admissions in developing settings.3
Data generated from several hospital cohorts had demonstrated that respiratory failure, followed by sepsis, and neurological diseases are the main causes of ICU admission among HIV-infected individuals.4–7 Respiratory failure accounts for more than 20% of all ICU admissions. Interestingly, in ART-treated individuals, the etiologies of respiratory failure are similar to those seen in general critically ill population (i.e. Streptococcus pneumoniae, sepsis, chronic obstructive pulmonary disease, and asthma).8,9
Sepsis accounts for 15%–30% of all ICU admissions of HIV-infected individuals.6.8,10,11 Nevertheless, HIV-infected patients with severe sepsis are less often admitted to ICU, even when compared to patients with similar clinical conditions or higher expected lethality.12 Studies undertaken in the current HAART era identified sepsis as an increasing cause of admission to ICU.8,10 Longer life span, more exposures to invasive procedures and higher organ dysfunction index at admission may partially explain this phenomenon.
The typical immunologic abnormalities associated with chronic HIV disease may predispose such individuals to systemic bacterial infections.13,14 Therefore, it has been proposed that sepsis may present with distinctive clinical, inflammatory, and prognostic features in this especial population.
HIV-associated sepsisClinical presentationThe main microbiology focus of infection mirrors those usually seen in uninfected individuals. Lung is the most common site of infection ranging from 52% to 72%.15–17 In two studies, the etiologies were primarily nosocomial flora: Gram-negative rods, such as Pseudomonas aeruginosa and Acinetobacter sps.; Gram-positive cocci, and fungi, especially, Candida sps.17,18 In contrast, others had found a predominance of fungal and mycobacterial infections in HIV-associated sepsis. The fungi identified were Pneumocystis jirovecii, Histoplasma capsulatum, Cryptococcus sp and Candida albicans.16 Of note, Haddy et al.19 reported more than 10% incidence rate of polymicrobial infections in HIV-related septicemia. Taken together, the data suggest that a wide empirical antimicrobial coverage is warranted in HIV septic patients. Further, the absence of classical clinical signs of sepsis in HIV-infected patients is a remarkable differential feature. Fever was absent in more than 45%. Pneumocystis jirovecy pneumonia (PCP) is the single most common AIDS-related infection, which contributes as a source of sepsis.15,16,18
HIV septic patients tend to spend more time in the ICU, are more severely ill (i.e. higher SAPS II, APACHE II, SOFA scores), most likely come from wards and needed invasive support and antibiotics more often. Although those characteristics may predispose infected individuals to acquisition of multidrug-resistant organisms, only catheter-related bacteremia has a higher incidence in the HIV-infected population admitted to ICU compared to controls.20
Inflammatory featuresAdvanced immunosuppression-associated with untreated HIV disease may blunt the systemic host response to infection; as a result, the levels of inflammatory biomarkers and the pathognomonic signs of infection might be profoundly depressed or even absent.21 Silva et al.16 showed that at admission septic HIV-infected patients presented with lower white blood counts, C-reactive protein (CRP), and Procalcitonin (PCT). In contrast, initial serum concentrations of IL-10 – a marker of immune dysfunction – were significantly higher in HIV-infected septic patients than in uninfected patients [4.4pg/mL (1.0–38.1) vs. 1.0pg/mL (1.0–2.7), respectively (p=0.005)]. In addition, Amancio et al.22 showed that serum IL-6 levels were significantly associated with mortality of HIV-infected patients with septic shock in a comparable fashion as seen in uninfected individuals. Interestingly, a tendency for persistently high levels of CRP and PCT was noted in non-survivors HIV septic patients. Therefore, serial monitoring of these acute phase reactants could probably identify those who would benefit most from intensive care support.
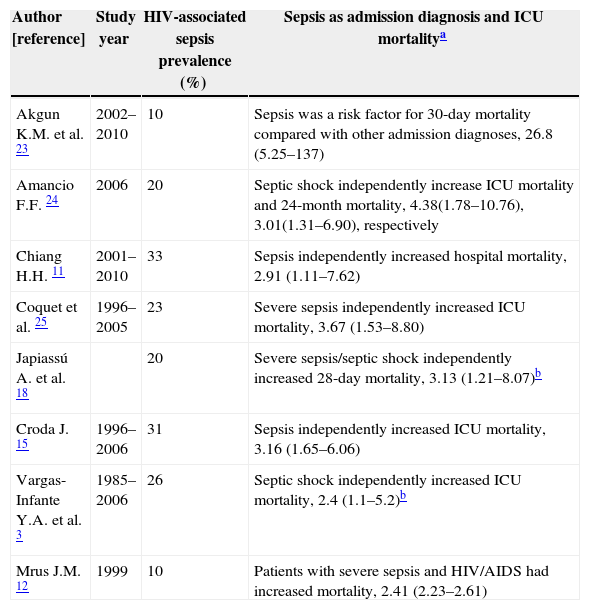
PrognosisThere is a robust high-quality data demonstrating a detrimental impact, both short-term and long-term, of HIV infection on sepsis (Table 1). It is estimated that in-ICU mortality rate of HIV-associated sepsis range from 29% to 76%. Similarly, those rates persist after six months of patient follow-up. Recently, Akgun23 showed that HIV-infected patients were more often admitted to ICU, particularly for respiratory and sepsis related diagnoses, and experienced higher 30-day mortality compared with uninfected patients. The detrimental impact of sepsis could not be solely attributed to immunological failure associated with HIV infection per se, because in the latter study more than 50% of infected subjects had viral suppression and mean CD4+ count were 281cells/mm3. It is speculated that residual immune dysregulation syndrome may play a crucial role in explaining this poor outcome, even in controlled HIV-infected patients with sepsis.26
Studies which HIV-associated sepsis was independently predictor of mortality.
| Author [reference] | Study year | HIV-associated sepsis prevalence (%) | Sepsis as admission diagnosis and ICU mortalitya |
|---|---|---|---|
| Akgun K.M. et al. 23 | 2002–2010 | 10 | Sepsis was a risk factor for 30-day mortality compared with other admission diagnoses, 26.8 (5.25–137) |
| Amancio F.F. 24 | 2006 | 20 | Septic shock independently increase ICU mortality and 24-month mortality, 4.38(1.78–10.76), 3.01(1.31–6.90), respectively |
| Chiang H.H. 11 | 2001–2010 | 33 | Sepsis independently increased hospital mortality, 2.91 (1.11–7.62) |
| Coquet et al. 25 | 1996–2005 | 23 | Severe sepsis independently increased ICU mortality, 3.67 (1.53–8.80) |
| Japiassú A. et al. 18 | 20 | Severe sepsis/septic shock independently increased 28-day mortality, 3.13 (1.21–8.07)b | |
| Croda J. 15 | 1996–2006 | 31 | Sepsis independently increased ICU mortality, 3.16 (1.65–6.06) |
| Vargas-Infante Y.A. et al. 3 | 1985–2006 | 26 | Septic shock independently increased ICU mortality, 2.4 (1.1–5.2)b |
| Mrus J.M. 12 | 1999 | 10 | Patients with severe sepsis and HIV/AIDS had increased mortality, 2.41 (2.23–2.61) |
Further, Mrus et al.12 showed that in a cohort of severe septic patients, those with HIV/AIDS had a statistically significant higher risk of mortality compared with uninfected severe septic patients (29% vs. 20%; p<0.0001). This risk persists after adjustments for potential confounders that could influence the outcome.
Few studies have evaluated the impact of sepsis in HIV-infected patients with suppressed viremia. Cobos-Trigueros et al.20 showed that sepsis was a predictor of in-ICU mortality, independently of HIV serological status, therefore, not just limited to the HIV-infected population. This finding is still difficult to explain, but it seems reasonable to believe that viral suppressed subjects might have the same hospital behavior patterns (etiologies, clinical features) found in septic uninfected patients.
Impact of antiretroviral therapyThe introduction of HAART has increased substantially in-hospital survival of critically ill HIV-infected patients in developed countries ranging from 43% to 80%. 27,28 In contrast, in developing settings those figures are lower, reflecting the high rates of late HIV presenters and opportunistic infections commonly seen in those places.15 Modern ICU standards of care unrelated to HIV therapy (i.e. early-goal therapy,29 prone position,30 low-tidal volume,31 intensive insulin therapy32) may explain itself the survival improvement, although studies designed to specifically assess the effect of those interventions are limited.
Whether or not the administration of HAART during ICU stay is beneficial remains a matter of continuous debate. Currently, randomized clinical trials evaluating this issue have not been performed yet. The data are extrapolated from studies enrolling participants in the non-ICU setting, less critically ill and not receiving critical support therapy such as mechanical ventilation and vasopressors. The generalizability of these results to critically ill HIV-infected patients must be done with caution. Furthermore, Huang et al. had already underscored the peculiar challenges related to antiretroviral use in hemodynamically unstable patients – bioavailability, drug interactions, immune reconstitution disease, and toxic effects.33
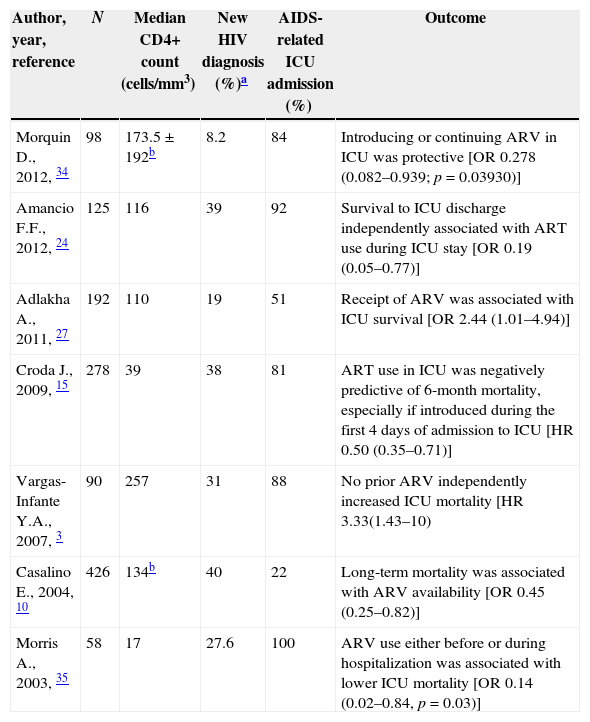
The cumulative data show no significant effect of HAART on short-term survival of critically ill HIV-infected patients, apart from seven studies that argue against it. Remarkably, most of these studies included a substantial proportion of HIV-late presenters, severely immunosuppressed with AIDS-related diagnosis at ICU admission (Table 2).
Studies in which ART administration during ICU was associated with improved hospital survival.
| Author, year, reference | N | Median CD4+ count (cells/mm3) | New HIV diagnosis (%)a | AIDS-related ICU admission (%) | Outcome |
|---|---|---|---|---|---|
| Morquin D., 2012, 34 | 98 | 173.5±192b | 8.2 | 84 | Introducing or continuing ARV in ICU was protective [OR 0.278 (0.082–0.939; p=0.03930)] |
| Amancio F.F., 2012, 24 | 125 | 116 | 39 | 92 | Survival to ICU discharge independently associated with ART use during ICU stay [OR 0.19 (0.05–0.77)] |
| Adlakha A., 2011, 27 | 192 | 110 | 19 | 51 | Receipt of ARV was associated with ICU survival [OR 2.44 (1.01–4.94)] |
| Croda J., 2009, 15 | 278 | 39 | 38 | 81 | ART use in ICU was negatively predictive of 6-month mortality, especially if introduced during the first 4 days of admission to ICU [HR 0.50 (0.35–0.71)] |
| Vargas-Infante Y.A., 2007, 3 | 90 | 257 | 31 | 88 | No prior ARV independently increased ICU mortality [HR 3.33(1.43–10) |
| Casalino E., 2004, 10 | 426 | 134b | 40 | 22 | Long-term mortality was associated with ARV availability [OR 0.45 (0.25–0.82)] |
| Morris A., 2003, 35 | 58 | 17 | 27.6 | 100 | ARV use either before or during hospitalization was associated with lower ICU mortality [OR 0.14 (0.02–0.84, p=0.03)] |
HAART use during ICU tended to be associated with less progression to AIDS-related events during the following 6-month follow-up period. A more robust immunologic recovery and rapidly declining of viral load during HAART use in-ICU may contribute to this result. In addition, Meybeck et al.36 have shown that in-ICU HAART use was associated with higher incidence of antiretroviral drug resistance with HAART use (25%) compared to 7% among those without p=0.02). Dynamic and complexes pharmacokinetics interactions involving antiretrovirals and common ICU medications, as well as erratic gastrointestinal absorption related to critical illness may explain the emergence of drug resistance in the HAART treated group.
In short, to date, HAART prescription for both, treatment-naïve or experienced patients cannot be routinely administered for HIV-associated sepsis. Only a sub-group of patients (i.e. those with AIDS-related OI) may benefit from HAART when administered soon after admission to ICU. Zolopa et al.37 have shown that in non-critically ill HIV-infected patients, those who started HAART within 2-weeks after OI treatment, had a lower rate of progress to AIDS or death. As a result, each case must be individualized, and possible benefits should outweigh the potential risks associated with the antiretroviral use.38
Treatment and preventionThe mainstay of therapy in HIV-associated sepsis should reflect the exact same priorities highlighted in several clinical guidelines.39 However, some issues must specifically be addressed in this setting in order to curb the huge burden of infection during sepsis. A significant proportion of patients are still unaware of the HIV diagnosis status at the time of ICU admission.15,18 Further, unnoticed AIDS diagnosis is associated with a poorer outcome. Consequently, efforts should be added, in order to ensure early HIV diagnosis and HIV-associated sepsis.
The fact that no single HIV-related factor (i.e. CD4+ count, VL) was shown to be directly associated with mortality during ICU stay could be a strong argument against widespread HAART use in ICU. Conversely, multiple factors associated with organ dysfunction at admission (mechanical ventilation use, APACHE II score) best explain the higher burden of septic HIV patients. For this reason, optimization of critical care bundles might be more beneficial as compared with HAART implementation in this special setting.
Whether enhanced vaccine programs for prevention of respiratory pathogens, improved engagement of care, broader use of HAART, and enhanced nosocomial-infection prevention protocols in ICU would improve care, and thus prevent HIV-associated sepsis are questions to be further elucidated.
ConclusionsCurrently, even with aggressive state of the art intensive care, HIV-associated sepsis remains a deadly entity, although substantial degrees of improvements have occurred over the years transforming HIV disease in a chronic condition. In order to ameliorate the extreme mortality associated with the condition, a future prospective randomized controlled trial needs to be designed to define optimal time to prescribe HAART in ICU (immediately vs. deferred approach). In particular, when to initiate antiretrovirals during HIV-associated sepsis should also be addressed. Whether or not a sub-group of patients would benefit from HAART introduced in ICU is still an elusive question. Besides, incorporation of antiretrovirals drug monitoring levels in routine ICU standard of care should also be investigated, because altered pharmacokinetic, caused by malabsorption, organ failure and drug interactions could place infected patients at risk of developing sub-therapeutic drug levels, and subsequently emerging drug resistance.
Finally, the development of bedside biomarkers is an intriguing evolving strategy, selecting those high-risk individuals who would be enrolled in future trials for sepsis.
Conflicts of interestThe author declares no conflicts of interest.
The author acknowledges Cristiane Lamas, Fernando Bozza and Andre Japiassú for proofreading the final manuscript.