In India, Elores (CSE-1034: ceftriaxone+sulbactam+disodium edetate) was approved as a broad spectrum antibiotic in year 2011 and is used for management of Extended Spectrum Beta Lactamases/Metallo Beta lactamases infections in tertiary care centers. The objective of this study was to investigate the efficacy of this drug in patients with Extended Spectrum Beta Lactamases/Metallo Beta lactamases infections and identify the incidence of adverse events in real clinical settings.
MethodsThis Post Marketing Surveillance study was conducted at 17 centers across India and included 2500 patients of all age groups suffering from various bacterial infections and treated with Elores (CSE1034). Information regarding demographic, clinical and microbiological parameters, dosage and treatment duration, efficacy and adverse events (AEs) associated with the treatment were recorded.
ResultsA total of 2500 patients were included in the study and efficacy was evaluated in 2487 patients. In total, 409 AEs were reported in 211 (8.4%) patients. The major AEs reported were vomiting (3.0%), pain at injection site (2.5%), nausea (2.3%), redness at site (1.96%), thrombophlebitis (1.4%). Of total reported AEs, 40 (5.3%) AEs were reported in pediatric, 310 (20.6%) in adult, and 59 (23.6%) in geriatric group. No AE belonging to grade IV or V was reported in any patient. In terms of efficacy, 1977 (79.4%) patients were cured, 501 (20.1%) patients showed clinical improvement and 5 (0.2%) patients were complete failure. The treatment duration varied from 5 to 7 days in different patients depending on the infection type.
ConclusionIn this post-marketing surveillance study, CSE-1034 was found to be an effective and safe option against Pip tazo and meropenem in management of patients with multi-drug resistant (MDR) bacterial infections under routine ward settings.
Antimicrobial resistance is one of the major issues faced globally posing a serious threat to the effective prevention and treatment of an ever-increasing range of resistant bacterial infections.1 The rapid pace of multi-drug-resistance (MDR) if left unattended can very soon land us in an era where common infections and minor injuries can become difficult to treat and end up as one of the prime causes of mortality. Although resistance trends vary among populations, there is significant evidence that the prevalence of MDR is increasing.2 According to WHO global report on surveillance, resistance in common bacterial pathogens has reached to alarming levels in many parts of the world and very few treatment options are effective against common infections in these regions.2 Infections due to MDR organisms can result in increased treatment costs, longer lengths of hospital stay and higher mortality rates.1,3
In recent years, a growing resistance among Gram-positive and Gram-negative pathogens causing community or hospital-based infections is on rise.4 The acronym “ESKAPE” has been coined for a faction of antibiotic-resistant pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumanii, Pseudomonas aeruginosa, and Enterobacter species) capable of escaping the biocidal effects of antimicrobial agents and are currently posing serious therapeutic dilemmas to medical practitioners.1 The mechanism of resistance among these infectious agents mainly include production of extended-spectrum beta-lactamases (ESBLs) and carbapenameses in Gram-negative pathogens, and due to mutant transpeptidase gene in Gram-positive organisms that have low affinity for beta-lactams.5,6 The production of these enzymes renders the current range of antibiotics which mainly include beta-lactamase inhibitors ineffective against these resistant pathogens. The biggest concern is that the drug of last resort for these infections is also reported to be repeatedly failing due to rise in development of resistance of the causative pathogens to these drugs and can very soon leave us with no choice of antibiotics for these MDR infections.5,6 Thus, all these studies argue for an augmentation of research on drug resistance and identify or develop new therapeutic strategies to widen the treatment horizon for effective infection control and antimicrobial use.
Elores, a novel Antibiotic Adjuvant Entity (AAE) of Ceftriaxone, Sulbactam and EDTA, herein referred as CSE-1034, has been recently developed and proposed as alternative option to solve the MDR menace to some extent. Due to synergistic action of inhibition of cell wall by ceftriaxone accompanied by the specific inhibition of beta-lactamases by beta-lactamase inhibitor component sulbactam and the non-antibiotic adjuvant EDTA acting as a catalyst and providing synergy to the combination. This drug has been reported to be effective against multiple type of MDR organisms.7,8 Clinical trials of CSE-1034 revealed that many MDR organisms resistant to other antibiotics, were sensitive to this novel AAE, which can serve as a potential alternative therapy to reduce the usage of currently available drugs like Pip tazo and carbapenems for these bacterial infections.9 This drug got approval from Indian FDA after phase III clinical trials and was there after marketed in India for the treatment of various bacterial infections. In order to check the efficacy and safety of this drug in real-world practice, a post marketing surveillance study was conducted immediately after CSE-1034 was launched in the market. In this article, we present the results of this post-marketing surveillance of CSE-1034 used in everyday clinical practice in a decent number of patients.
Materials and methodsStudy designThis study was designed to investigate the safety and efficacy of CSE-1034 in department (IPD) inpatients with mild to severe bacterial infections including lower respiratory tract infection (LRTI), urinary tract infection (UTI), bacterial meningitis, bone & joint infection, bacterial sepsis, surgical infection, skin and soft tissue infections and enteric fever. A total of 2500 patients were recruited from 17 medical centers across India from September 2011 to July 2015, and treatment was based on the decision of the physician. The study was carried out in accordance with “Guidelines for Clinical Trials on Pharmaceutical Products in India GCP Guidelines” and the ethical principles enunciated in the Declaration of Helsinki. The study was approved by the independent ethics committee (IEC)/institutional review board (IRB) before commencement. Only patients who had given written informed consent were included in the study.
Study subjectsThe main criteria for patient inclusion in the study were: (1) the primary diagnosis of bacterial infections based on clinical history, signs, symptoms and laboratory investigations, and judged by the treating physician empirically; and (2) subjects with negative culture report were enrolled based on their other supportive investigations like chest-X ray in LRTI or as per clinical judgment of study investigators. Exclusion criteria included (1) subjects with history of hypersensitivity, allergic response or any contraindications to cephalosporin group of drugs or CSE-1034; (2) subject undergoing treatment with other active drug of the cephalosporin class; (3) history of hearing loss; and (4) pregnant or lactating women.
In vitro microbial antibiotic-susceptibility testingKirby–Bauer disk diffusion method was used to test the antimicrobial susceptibility of the pathogen isolated from the patients.
Antibiotic dosageThe dose of CSE-1034 given to adults was 1.5g every 12h and the dose for pediatric group varied from 30 to 75mg/kg of body weight depending on the type and severity of infection (up to 120mg/kg body weight in severe infections).
Study design and analysisStudy itemsInformation regarding demographic and baseline characters of each patient, susceptibility profile the dosage and treatment duration, concomitant medications, and AEs associated with the treatment were recorded.
SafetyAEs were defined as any untoward medical occurrence taking place during or after treatment with the drug. AEs whether treatment related or not were decided by the physicians. The seriousness of AEs was determined as per the ICH-E2D guidelines and the AEs data were compiled according to the ICH Medical Dictionary for Regulatory Activities.
EfficacyOut of 2500 IPD patients, 2483 completed the treatment and 17 subjects were not included in efficacy analysis due to treatment period of less than three days. The clinical response of the therapy was evaluated in terms of improvement in clinical parameters on the day of treatment closure of the treatment plan. Clinical responses were evaluated on the basis of changes in clinical signs/symptoms and were categorized as “cure”, “failure”, or “improved”.
Various hematological and biochemical investigations including hemoglobin test, ESR, total leukocyte count (TLC), differential leukocyte count (DLC), liver function test (LFT), kidney function test (KFT) were carried out at the beginning and the end of treatment to evaluate the drug safety and efficacy.
Statistical analysisAll statistical analyses were performed using chi-square test. p-Values were two-tailed and a value of <0.05 was considered as statistically significant.
ResultsBaseline demographic data of all the patientsOut of 2500 IPD patients, 750 (30%) were pediatric patients with a mean age of 11.85±3.28, 250 (10%) belonged to geriatric age group with a mean age of 73.70±6.14 years, and 1500 (60%) were adults with a mean age of 41.55±13.74 years. Based on the type of infection, the patient disposition was, 1301 (52.04%) LRTI, 597 (23.88%) UTI, 178 (7.12%) surgical infections, 185 (7.4%) enteric fever, 135 (5.4%) bacterial sepsis, 55 (2.2%) bacterial meningitis, 35 (1.4%) bone & joint infection, and 14 (0.56%) skin & soft tissue infection.
Of the total 750 pediatric patients, 265 (35%) had LRTI, 214 (28.5%) UTI, 89 (11.8%) enteric fever, 87 (11.6%) sepsis, 39 (5.2%) surgical infections, 37 (4.9%) bacterial meningitis, and 19 (2.5%) had bone and joint infections. The distribution of adult age group on the basis of infection were 924 (61.6%) of LRTI, 321 (21%) of UTI, 97 (6.5%) of surgical infections, 88 (5.9%) of enteric fever, 37 (2.5%) of sepsis, 13 (0.9%) of meningitis, 11 (0.73%) of skin and soft tissues infections, and 9 (0.6%) of bone and joint infections. In geriatric age group, the pattern observed was 112 (45%) LRTI, 62 (25%) UTI, 42 (17%) surgical infections, 11 (4.4%) sepsis, 8 (3.2%) enteric fever, 7 (2.8%) bone and joint infections, 5 (2%) meningitis, and 3 (1.2%) skin and soft tissue infections.
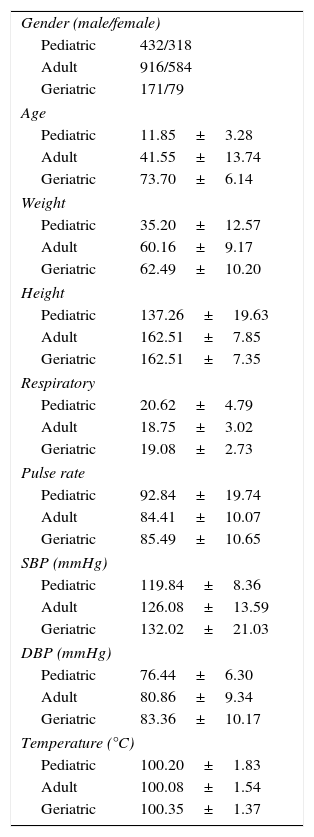
Demographic analysis data for other parameters like age, weight, height, respiration rate, pulse rate, SBP, DBP, and temperature is mentioned in detail in Table 1. All the analyzed patients met the inclusion and exclusion criteria of approved protocol.
Distribution of baseline patient characteristics.
| Gender (male/female) | |
| Pediatric | 432/318 |
| Adult | 916/584 |
| Geriatric | 171/79 |
| Age | |
| Pediatric | 11.85±3.28 |
| Adult | 41.55±13.74 |
| Geriatric | 73.70±6.14 |
| Weight | |
| Pediatric | 35.20±12.57 |
| Adult | 60.16±9.17 |
| Geriatric | 62.49±10.20 |
| Height | |
| Pediatric | 137.26±19.63 |
| Adult | 162.51±7.85 |
| Geriatric | 162.51±7.35 |
| Respiratory | |
| Pediatric | 20.62±4.79 |
| Adult | 18.75±3.02 |
| Geriatric | 19.08±2.73 |
| Pulse rate | |
| Pediatric | 92.84±19.74 |
| Adult | 84.41±10.07 |
| Geriatric | 85.49±10.65 |
| SBP (mmHg) | |
| Pediatric | 119.84±8.36 |
| Adult | 126.08±13.59 |
| Geriatric | 132.02±21.03 |
| DBP (mmHg) | |
| Pediatric | 76.44±6.30 |
| Adult | 80.86±9.34 |
| Geriatric | 83.36±10.17 |
| Temperature (°C) | |
| Pediatric | 100.20±1.83 |
| Adult | 100.08±1.54 |
| Geriatric | 100.35±1.37 |
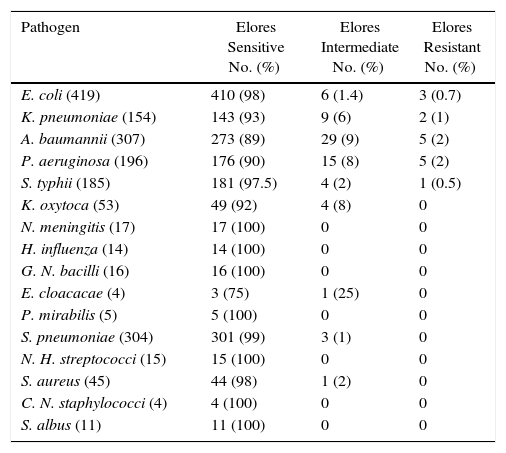
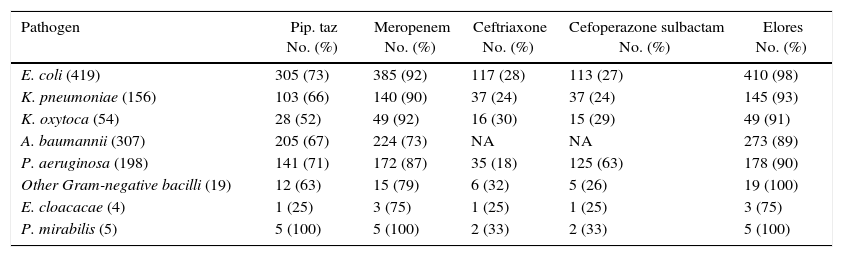
E. coli was shown to be the predominant pathogen isolated in 419 patients followed by A. baumannii in 307, S. pneumoniae in 304, P. aeruginosa in 196, K. pneumoniae in 154, S. typhii in 185, K. oxytoca in 53, S. aureus in 45, N. meningitis in 17, Gram-negative bacilli in 16, H. influenza in 14, non-hemolytic streptococci in 15, S. albus in 11, P. mirabilis in 5, Coagulase-negative staphylococci in 4, and E. cloaceae in 4. Mixed growth was observed in 84 and no growth was observed in 657 patients. The susceptibility profile to CSE-1034 of the pathogens isolated is shown in Table 2 and to other antibiotics is detailed in Table 3. Susceptibility of CSE1034 was far more superior to Pip Tazo, Cefoperazone Sulbactam and was at par with Meropenem.
In vitro antibiotic susceptibility testing of AAE for pathogens isolated from single organism infections.
| Pathogen | Elores Sensitive No. (%) | Elores Intermediate No. (%) | Elores Resistant No. (%) |
|---|---|---|---|
| E. coli (419) | 410 (98) | 6 (1.4) | 3 (0.7) |
| K. pneumoniae (154) | 143 (93) | 9 (6) | 2 (1) |
| A. baumannii (307) | 273 (89) | 29 (9) | 5 (2) |
| P. aeruginosa (196) | 176 (90) | 15 (8) | 5 (2) |
| S. typhii (185) | 181 (97.5) | 4 (2) | 1 (0.5) |
| K. oxytoca (53) | 49 (92) | 4 (8) | 0 |
| N. meningitis (17) | 17 (100) | 0 | 0 |
| H. influenza (14) | 14 (100) | 0 | 0 |
| G. N. bacilli (16) | 16 (100) | 0 | 0 |
| E. cloacacae (4) | 3 (75) | 1 (25) | 0 |
| P. mirabilis (5) | 5 (100) | 0 | 0 |
| S. pneumoniae (304) | 301 (99) | 3 (1) | 0 |
| N. H. streptococci (15) | 15 (100) | 0 | 0 |
| S. aureus (45) | 44 (98) | 1 (2) | 0 |
| C. N. staphylococci (4) | 4 (100) | 0 | 0 |
| S. albus (11) | 11 (100) | 0 | 0 |
In vitro susceptibility profile to various antibiotics for pathogens isolated from single organism infections.
| Pathogen | Pip. taz No. (%) | Meropenem No. (%) | Ceftriaxone No. (%) | Cefoperazone sulbactam No. (%) | Elores No. (%) |
|---|---|---|---|---|---|
| E. coli (419) | 305 (73) | 385 (92) | 117 (28) | 113 (27) | 410 (98) |
| K. pneumoniae (156) | 103 (66) | 140 (90) | 37 (24) | 37 (24) | 145 (93) |
| K. oxytoca (54) | 28 (52) | 49 (92) | 16 (30) | 15 (29) | 49 (91) |
| A. baumannii (307) | 205 (67) | 224 (73) | NA | NA | 273 (89) |
| P. aeruginosa (198) | 141 (71) | 172 (87) | 35 (18) | 125 (63) | 178 (90) |
| Other Gram-negative bacilli (19) | 12 (63) | 15 (79) | 6 (32) | 5 (26) | 19 (100) |
| E. cloacacae (4) | 1 (25) | 3 (75) | 1 (25) | 1 (25) | 3 (75) |
| P. mirabilis (5) | 5 (100) | 5 (100) | 2 (33) | 2 (33) | 5 (100) |
| Pathogen | Ceftriaxone No (%) | Ticarcillin+clavulanic acid No (%) | Ciprofloxacin No (%) | Linezolid No (%) | Elores No (%) |
|---|---|---|---|---|---|
| S. pneumoniae (304) | 298 (98) | 283 (93) | 237 (78) | 304 (100) | 300 (99) |
| N. H. streptococci (15) | 15 (100) | 15 (100) | 11 (74) | 15 (100) | 15 (100) |
| N. meningitis (17) | 17 (100) | 17 (100) | 14 (80) | 17 (100) | |
| H. influenza (14) | 14 (100) | 13 (93) | 13 (93) | 14 (100) |
| Pathogen | Amoxycillin clavulanic acid No (%) | Cefoxitin No (%) | Linezolid No (%) | Teicoplanin No (%) | Elores No (%) |
|---|---|---|---|---|---|
| S. aureus (45) | 30 (67) | 45 (100) | 45 (100) | 45 (100) | 44 (98) |
| C. N. staphylococci (4) | 3 (75) | 4 (100) | 4 (100) | 4 (100) | 4 (100) |
| S. albus (12) | 8 (66) | 12 (100) | 12 (100) | 12 (100) | 12 (100) |
| Pathogen | Ceftriaxone No (%) | Nalidixic acid No (%) | Ampicillin No (%) | Chloramphenicol No (%) | Elores No (%) |
|---|---|---|---|---|---|
| S. typhii (185) | 174 (94) | 131 (71) | 40 (22) | 35 (19) | 181 (98) |
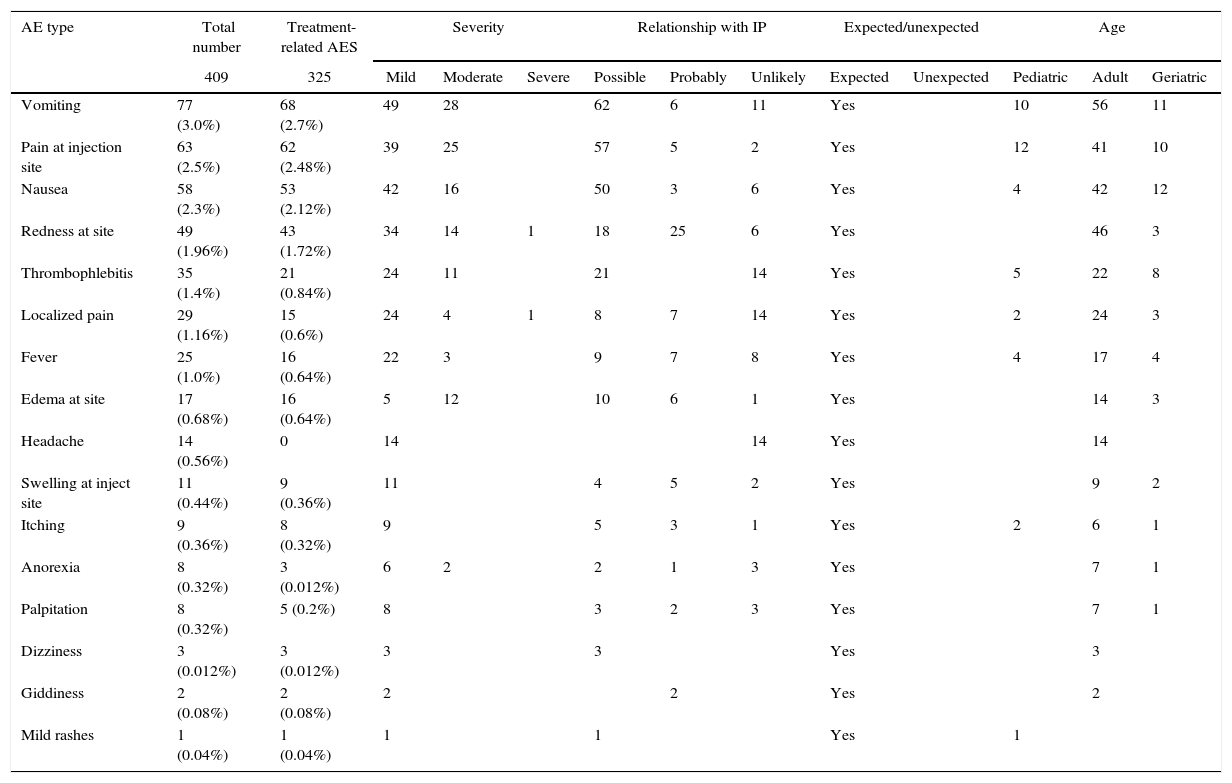
In total, 409 AEs of all grades were reported in 211 patients. The most common AEs were vomiting (3.0%), pain at injection site (2.5%), nausea (2.3%), redness at site (1.96%), thrombophlebitis (1.4%), localized pain (1.16%), fever (1.0%), edema (0.68%), headache (0.56%), swelling (0.44%), itching (0.36%), anorexia (0.32%), palpitation (0.32%), dizziness (0.012%), giddiness (0.08%), and mild rashes (0.04%). For details refer to Table 4.
Most commonly reported AEs in 409 patients with various bacterial infections sorted by different parameters.
| AE type | Total number | Treatment-related AES | Severity | Relationship with IP | Expected/unexpected | Age | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 409 | 325 | Mild | Moderate | Severe | Possible | Probably | Unlikely | Expected | Unexpected | Pediatric | Adult | Geriatric | |
| Vomiting | 77 (3.0%) | 68 (2.7%) | 49 | 28 | 62 | 6 | 11 | Yes | 10 | 56 | 11 | ||
| Pain at injection site | 63 (2.5%) | 62 (2.48%) | 39 | 25 | 57 | 5 | 2 | Yes | 12 | 41 | 10 | ||
| Nausea | 58 (2.3%) | 53 (2.12%) | 42 | 16 | 50 | 3 | 6 | Yes | 4 | 42 | 12 | ||
| Redness at site | 49 (1.96%) | 43 (1.72%) | 34 | 14 | 1 | 18 | 25 | 6 | Yes | 46 | 3 | ||
| Thrombophlebitis | 35 (1.4%) | 21 (0.84%) | 24 | 11 | 21 | 14 | Yes | 5 | 22 | 8 | |||
| Localized pain | 29 (1.16%) | 15 (0.6%) | 24 | 4 | 1 | 8 | 7 | 14 | Yes | 2 | 24 | 3 | |
| Fever | 25 (1.0%) | 16 (0.64%) | 22 | 3 | 9 | 7 | 8 | Yes | 4 | 17 | 4 | ||
| Edema at site | 17 (0.68%) | 16 (0.64%) | 5 | 12 | 10 | 6 | 1 | Yes | 14 | 3 | |||
| Headache | 14 (0.56%) | 0 | 14 | 14 | Yes | 14 | |||||||
| Swelling at inject site | 11 (0.44%) | 9 (0.36%) | 11 | 4 | 5 | 2 | Yes | 9 | 2 | ||||
| Itching | 9 (0.36%) | 8 (0.32%) | 9 | 5 | 3 | 1 | Yes | 2 | 6 | 1 | |||
| Anorexia | 8 (0.32%) | 3 (0.012%) | 6 | 2 | 2 | 1 | 3 | Yes | 7 | 1 | |||
| Palpitation | 8 (0.32%) | 5 (0.2%) | 8 | 3 | 2 | 3 | Yes | 7 | 1 | ||||
| Dizziness | 3 (0.012%) | 3 (0.012%) | 3 | 3 | Yes | 3 | |||||||
| Giddiness | 2 (0.08%) | 2 (0.08%) | 2 | 2 | Yes | 2 | |||||||
| Mild rashes | 1 (0.04%) | 1 (0.04%) | 1 | 1 | Yes | 1 | |||||||
The total number of AEs reported in pediatric, adult, and geriatric treatment groups were 40 (5.3%), 310 (20.6%), and 59 (23.6%), respectively. In the pediatric group six (2.2%) AEs were reported in patients with LRTI, four (1.86%) with UTI, seven (17.9%) with surgical infections, nine (10.3%) with bacterial sepsis, seven (7.86%) with enteric fever, five (5.6%) with bacterial meningitis, and two (10.5%) with bone and joint infections.
In the adult group, 145 (15.7%) AEs were reported in patients with LRTI, 63 (19.6%) with UTI, 32 (33%) with surgical infections, 30 (81%) with bacterial sepsis, 23 (26%) with enteric fever, eight (61.5%) with bacterial meningitis, five (45%) with soft and skin tissue infections, and four (44%%) with bone and joint infections. In the geriatric group, 12 (10.7%) AEs were reported in patients with LRTI, 21 (33.8%) with UTI, eight (19%) with surgical infections, six (54.8%) with bacterial sepsis, six (75%) with enteric fever, three (60%) with bacterial meningitis, and three (33%) with bone and joint infections.
Furthermore, classifying the AEs on the basis of organ system involved, 179 AEs were related to general disorder and site conditions, 143 to gastrointestinal system, 60 to vascular system, 19 to central nervous system, and eight AEs were related to cardiovascular system.
Intensity and the relationship of AEsBased on severity, 292 AEs were reported as mild in intensity (Grade I), 114 as moderate (Grade II), and two were reported as severe (Grade III). There was no report of grade IV or V AE in any of the patients. All the reported AEs were adverse drug reactions (ADRs) and were expected (Table 4). Two ADRs of severe grade included redness at site and localized pain.
In the pediatric group, 35 (4.66%) AEs were mild and five (0.66%) were moderate in severity; no severe AE was reported. In the adult Group, 222 (14.80%) reported AEs were mild in severity, 86 (5.73%) moderate, and two (0.13%) severe in intensity. However, in the geriatric group, 35 (14.0%) were mild and 24 (9.60%) moderate whereas no severe AE was reported by the prescribing physician (Table 4).
Based on the relationship with the investigational product (IP), 252 AEs were found to have possible relationship, 72 to have probable relationship, and 85 unlikely related to drug (Table 4). Thus overall, 325 treatment-related AEs were reported.
Overall assessment of the drug tolerability has shown it to have excellent tolerance in 1133 (45.6%), tolerable at a good level in 1319 (53.2%), fairly tolerable in 26 (1.1%), and not well-tolerated in five (0.2%).
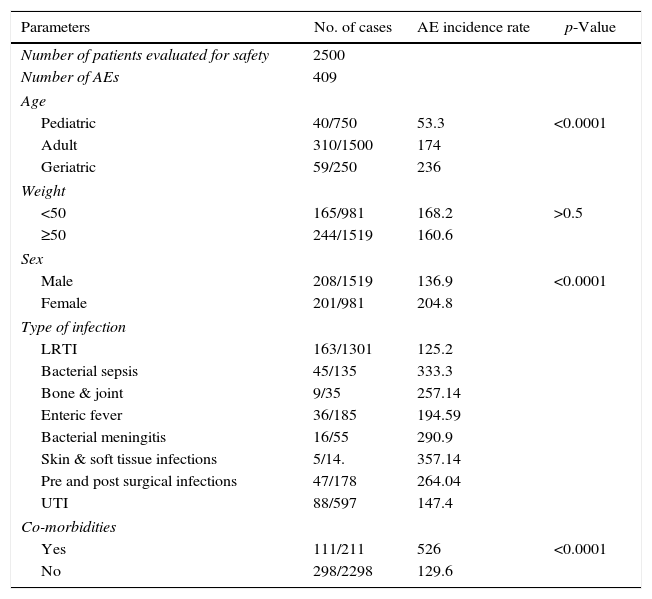
Risk factors for AEsTable 5 depicts the correlation between AEs incidence with the various background factors. From the analysis, patients in the “age group of ≥65” were seen to have significantly higher AE incidence compared to the pediatric and adult age groups (p-value >0.001). Significant differences were also observed between the patients in different weight groups. Patients in the weight group of “≥50” had significantly higher number of AEs compared to the group weighing less than 50kg (p-value >0.0001). However, there were no significant differences between categories in terms of gender.
Risk factors for the occurrence of AEs.
| Parameters | No. of cases | AE incidence rate | p-Value |
|---|---|---|---|
| Number of patients evaluated for safety | 2500 | ||
| Number of AEs | 409 | ||
| Age | |||
| Pediatric | 40/750 | 53.3 | <0.0001 |
| Adult | 310/1500 | 174 | |
| Geriatric | 59/250 | 236 | |
| Weight | |||
| <50 | 165/981 | 168.2 | >0.5 |
| ≥50 | 244/1519 | 160.6 | |
| Sex | |||
| Male | 208/1519 | 136.9 | <0.0001 |
| Female | 201/981 | 204.8 | |
| Type of infection | |||
| LRTI | 163/1301 | 125.2 | |
| Bacterial sepsis | 45/135 | 333.3 | |
| Bone & joint | 9/35 | 257.14 | |
| Enteric fever | 36/185 | 194.59 | |
| Bacterial meningitis | 16/55 | 290.9 | |
| Skin & soft tissue infections | 5/14. | 357.14 | |
| Pre and post surgical infections | 47/178 | 264.04 | |
| UTI | 88/597 | 147.4 | |
| Co-morbidities | |||
| Yes | 111/211 | 526 | <0.0001 |
| No | 298/2298 | 129.6 | |
The incidence of “AEs” was also observed to be significantly higher in patients with co-morbidities then in those with no co-morbidities (p-value >0.0001). Similarly, a positive association although not significant was observed between different type of infections and the AEs reported. Patients with soft and skin tissue infections and those with bacterial sepsis presented more AEs than patients with bacterial meningitis, pre- and post-surgical infections, bone and joint infections, LRTI, UTI, and enteric fever. Most of the AEs observed were related to the drug class and no new AE was observed.
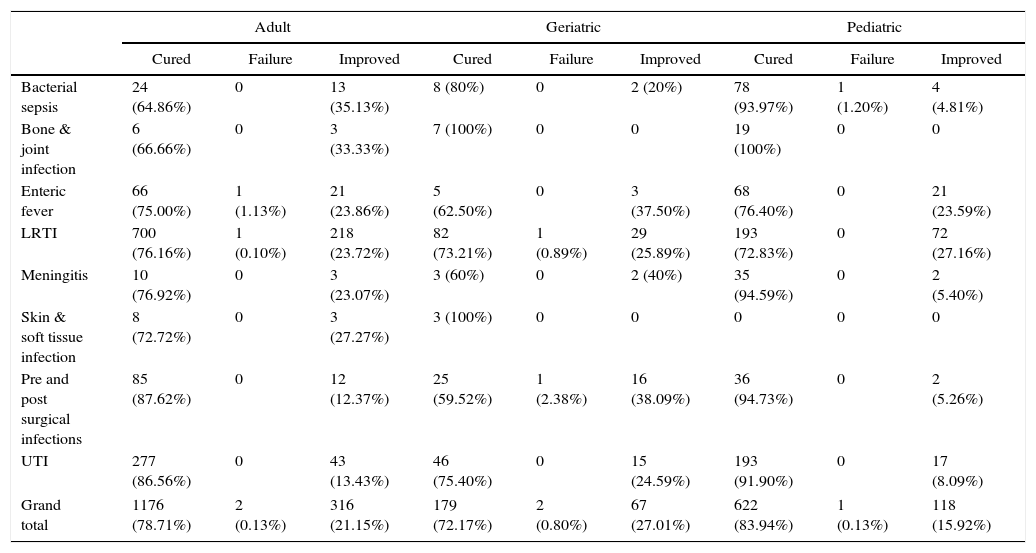
Clinical responseEfficacyIn the pediatric group of 741 patients, 622 patients (83.95%) were cured, 118 patient (15.92%) showed clinical improvement and one patient (0.13%) had treatment failure. Of 1494 adults, 1176 (78.72%) were cured, 316 (21.15%) showed clinical improvement, and two patients (0.13%) had complete treatment failure. In the geriatric group of 248 patients, 179 (72.18%) were clinically cured, 67 patients (27.02%) showed improvement, and two patients (0.80%) had treatment failure (Table 6). In total, 1977 patients were cured, 501 showed clinical improvement and five had complete failure.
Display of outcomes according to age groups.
| Adult | Geriatric | Pediatric | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cured | Failure | Improved | Cured | Failure | Improved | Cured | Failure | Improved | |
| Bacterial sepsis | 24 (64.86%) | 0 | 13 (35.13%) | 8 (80%) | 0 | 2 (20%) | 78 (93.97%) | 1 (1.20%) | 4 (4.81%) |
| Bone & joint infection | 6 (66.66%) | 0 | 3 (33.33%) | 7 (100%) | 0 | 0 | 19 (100%) | 0 | 0 |
| Enteric fever | 66 (75.00%) | 1 (1.13%) | 21 (23.86%) | 5 (62.50%) | 0 | 3 (37.50%) | 68 (76.40%) | 0 | 21 (23.59%) |
| LRTI | 700 (76.16%) | 1 (0.10%) | 218 (23.72%) | 82 (73.21%) | 1 (0.89%) | 29 (25.89%) | 193 (72.83%) | 0 | 72 (27.16%) |
| Meningitis | 10 (76.92%) | 0 | 3 (23.07%) | 3 (60%) | 0 | 2 (40%) | 35 (94.59%) | 0 | 2 (5.40%) |
| Skin & soft tissue infection | 8 (72.72%) | 0 | 3 (27.27%) | 3 (100%) | 0 | 0 | 0 | 0 | 0 |
| Pre and post surgical infections | 85 (87.62%) | 0 | 12 (12.37%) | 25 (59.52%) | 1 (2.38%) | 16 (38.09%) | 36 (94.73%) | 0 | 2 (5.26%) |
| UTI | 277 (86.56%) | 0 | 43 (13.43%) | 46 (75.40%) | 0 | 15 (24.59%) | 193 (91.90%) | 0 | 17 (8.09%) |
| Grand total | 1176 (78.71%) | 2 (0.13%) | 316 (21.15%) | 179 (72.17%) | 2 (0.80%) | 67 (27.01%) | 622 (83.94%) | 1 (0.13%) | 118 (15.92%) |
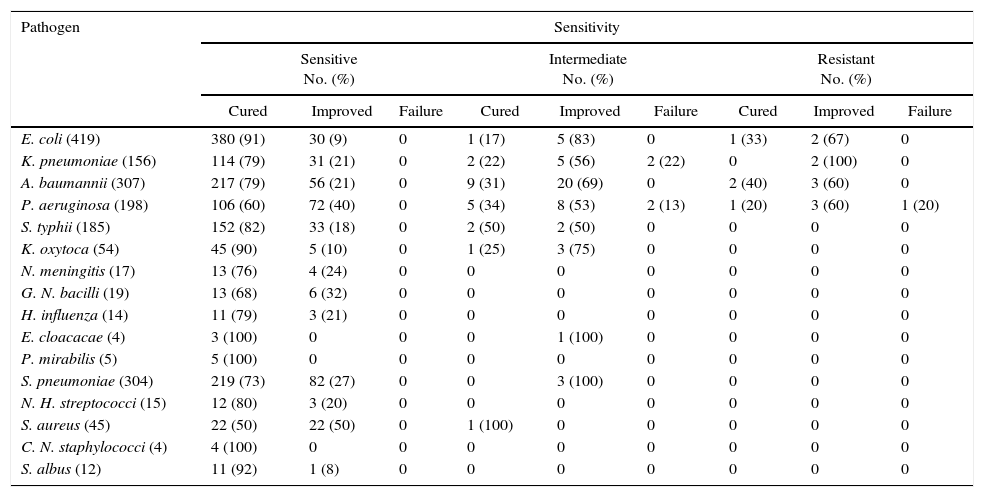
The clinical response based on the type of pathogen isolated is summarized in Table 7. The highest cure rate among the predominant pathogens was observed with E. coli (91%) followed by K. pneumoniae (79), A. baumannii (79%), and S. pneumoniae (73%).
Display of clinical outcome according to the type of pathogen isolated.
| Pathogen | Sensitivity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitive No. (%) | Intermediate No. (%) | Resistant No. (%) | |||||||
| Cured | Improved | Failure | Cured | Improved | Failure | Cured | Improved | Failure | |
| E. coli (419) | 380 (91) | 30 (9) | 0 | 1 (17) | 5 (83) | 0 | 1 (33) | 2 (67) | 0 |
| K. pneumoniae (156) | 114 (79) | 31 (21) | 0 | 2 (22) | 5 (56) | 2 (22) | 0 | 2 (100) | 0 |
| A. baumannii (307) | 217 (79) | 56 (21) | 0 | 9 (31) | 20 (69) | 0 | 2 (40) | 3 (60) | 0 |
| P. aeruginosa (198) | 106 (60) | 72 (40) | 0 | 5 (34) | 8 (53) | 2 (13) | 1 (20) | 3 (60) | 1 (20) |
| S. typhii (185) | 152 (82) | 33 (18) | 0 | 2 (50) | 2 (50) | 0 | 0 | 0 | 0 |
| K. oxytoca (54) | 45 (90) | 5 (10) | 0 | 1 (25) | 3 (75) | 0 | 0 | 0 | 0 |
| N. meningitis (17) | 13 (76) | 4 (24) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| G. N. bacilli (19) | 13 (68) | 6 (32) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H. influenza (14) | 11 (79) | 3 (21) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| E. cloacacae (4) | 3 (100) | 0 | 0 | 0 | 1 (100) | 0 | 0 | 0 | 0 |
| P. mirabilis (5) | 5 (100) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. pneumoniae (304) | 219 (73) | 82 (27) | 0 | 0 | 3 (100) | 0 | 0 | 0 | 0 |
| N. H. streptococci (15) | 12 (80) | 3 (20) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. aureus (45) | 22 (50) | 22 (50) | 0 | 1 (100) | 0 | 0 | 0 | 0 | 0 |
| C. N. staphylococci (4) | 4 (100) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. albus (12) | 11 (92) | 1 (8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
The treatment duration varied depending on the type of infection. The lowest treatment duration of five days was reported for LRTI and the highest of 10 days was for patients with infections of skin and soft tissue, bone and joint, and bacterial sepsis. For patients with UTI, bacterial meningitis, enteric fever, pre- and post-surgical infections, the mean treatment duration was 7.5 days.
DiscussionThe clinical trials for drugs are usually conducted in controlled environment with a number of limiting factors including the number of patients enrolled, age, associated co-morbidities, and medical history. Thus, the information retrieved through clinical trials is not the right way to predict drug effectiveness and safety in the real-world settings. Therefore, it becomes very important to collect and evaluate the drug further after being launched in the market. One of the recent drugs that was launched in market in 2011 as a potential treatment option for various bacterial infections was CSE-1034. This PMS study was undertaken to evaluate the safety and efficacy of CSE-1034 for the treatment of various MDR bacterial infections in IPD population under routine clinical settings.
Most of the patients evaluated in this study were adults aged 18–65 (60%) followed by pediatric (30%) and geriatric (10%) patients. LRTI was observed to be the most common (52%) infection followed by UTI (23.9%), enteric fever (7.4%), surgical infections (7.12%), bacterial sepsis (5.4%), bacterial meningitis (2.2%), bone and joints infection (1.4%), and skin and soft tissue infections (0.56%), well in accordance with various previous studies that have demonstrated a similar kind of infection pattern.10 The most frequent cause of these bacterial infections was E. coli (17%) followed by A. baumannii (12%), S. pneumoniae (12%), P. aeruginosa (8%), S. typhii (7%), and K. pneumoniae (6%). Overall, the Gram-negative pathogens were predominant as compared to Gram-positive pathogens.
Evaluating the drug in terms of safety, 409 AEs were reported in 211 patients accounting to 8.4% incidence. The common AEs reported were vomiting (3.0%), pain at injection site (2.5%), nausea (2.3%), redness at site (1.96%), thrombophlebitis (1.4%), and fever (1.0%). All reported AEs are known to be commonly associated with the antibiotic treatment of these bacterial infections. The most common systems affected by AEs were skin manifestations and general disorders followed by GI, vascular, and nervous system disorders. Consistent with our observations, a multitude of previous reports described a similar kind of pattern of organ/systems associated with AEs with various anti-bacterial drugs.11,12
When compared to the incidence of ADRs associated with other drugs of choice for multi-drug resistant infections, the incidence of ADRs was lower for this novel antibiotic Adjuvant Entity. In a meta-analysis study on the adverse effects of doripenem and other comparative drugs for the treatment of various bacterial infections 21–23% of the patients reported AEs for all the drugs compared.13 Similarly, a multi-center study evaluating and comparing the efficacy of meropenem to cefuroxime-gentamicin (±metronidazole) for the treatment of serious bacterial infections in elderly patients reported treatment-related AEs in 48.7% in meropenem-treated patients and in 45% with combination therapy14 (12). Likewise, in another similar kind of safety analysis study, treatment-related AEs were reported in 64% of patients receiving dorepenem and 60% of the patients receiving levofloxacin for the treatment of UTI.15 Furthermore, these studies have also reported that around 5–10% serious AEs were associated with the treatment.13,15 In contrast, no treatment-related ADRs belonging to grade IV or V was reported in any of the patients in our study. Furthermore, it should be emphasized that age-wise comparison of AEs has shown that the lowest number of AEs were reported in pediatric patients, thus it can also be concluded that this drug can be safely used in children as well. ADRs are one the main causes of healthcare-associated morbidity and mortality.16 In US, an estimated 7000 deaths occur due to ADRs making it the fourth leading cause of death in US.17 Not only do ADRs cause death and injury but they also affect the length of stay in hospitals which in turn lead to increased healthcare costs and decreased patient productivity.18 Thus overall, low incidence of AEs associated with this drug particularly in pediatric patients makes it an ideal alternative in terms of lower mortality rate and lower financial burdens associated with it.
Evaluating the risk factors for AEs, three factors including age, sex, and associated co-morbidities were observed to be significantly related to the occurrence of ADRs. Overall AEs reported in this study were more common in the geriatric group compared to the pediatric and adult groups. This kind of drug behavior could possibly be associated with decreased drug tolerance, reduced capacity to metabolize19 and eliminate drugs,20 and multiple medical problems associated with the growing age. Supporting our data, a study by Debellis et al.21 has shown that the incidences of ADRs are common and often preventable among older persons in the ambulatory clinical settings. Similarly, various other studies have shown that older people are more than twice as susceptible to ADRs as younger people.22,23 The increased rate of AEs among females could possibly be attributed to anatomical and physiological differences including body weight, body fat, gastrointestinal factors, menopause, menstruation, etc. which could affect pharmacokinetics and pharmacodynamics of drugs in the body.24,25 A group of studies have reported pronounced differences in the incidences of ADRs between two genders.16,26 The rate of AEs were also reported to be higher in patients with associated co-morbidities indicating that concomitant disease may also influence susceptibility to ADRs. In agreement with our results, various studies have shown that co-morbidities can form a very important factor leading to drug–disease interaction and ADRs.27,28 Commonly associated co-morbidities that have been widely shown to increase treatment-related AEs due to enhanced drug–disease interactions include diabetes, irregular blood pressure, insomnia, ulcer, glaucoma, enlarged prostate, and poor bladder control.27,28
This PMS study also demonstrated that CSE-1034 is an effective treatment in IPD patients with various MDR bacterial infections with cure rates in 79.62% of the patients and clinical improvement seen in 20.17% of the patients. In the subgroup analysis based on age, pediatric patients had the highest clinical response rate with 84% reporting total cure and 16% reporting clinical improvement; the cure rate of adults was 78% and clinical improvement 21%. Geriatric patients had a 72% cure rate and 27% clinical improvement. Infections caused by E. coli had the highest cure rate of 91% followed by 79% of infections by K. pneumoniae, 79% by A. baumannii, and 73% by S. pneumoniae. The results of clinical cur rate were in accordance with microbial susceptibility. The clinical response rates of CSE-1034 observed in this study are consistent with the results of prior studies conducted with this novel antibiotic to evaluate its effectiveness.7,29 Currently, the widely used class of drugs to treat adult and pediatric patients with serious MDR infections includes the penem family. When compared to the comparator drugs, the clinical cure rate with this drug is at par or in some cases better than the best available drugs of choice in the market for MDR infections.13,30
Thus, these results overall suggests that CSE-1034 is a valuable alternative to penems because of several advantages like the low rate of ADRs events. Secondly, no serious grade III or IV AE was associated with it. Thirdly, the clinical effectiveness of this drug was comparable to other drugs because patients who did not respond to Pip tazo and Cefoperazone sulbactam responded well on CSE1034. Therefore, CSE1034 can help us to minimize the usage of penems and spare them for the situations where they are actually demanded.
ConclusionsThe CSE-1034 was well-tolerated and effective for IPD patients of all age groups having different MDR bacterial infections. The low rate of ADRs associated with the drug along with the high clinical effectiveness make it an ideal alternative for the treatment of various bacterial infections.
Conflicts of interestThe authors declare no conflicts of interest.
Dr Mahip Saluja (Subharti Medical College & Hospital, Meerut, U.P.), Dr Parag Sharma (Devnandi Hospital, Hapur, U.P.), Dr Arvind jain (Dr Arvind Jains's clinic, Agra, U.P.), Dr Sunita Singh (K. K. hospital, Lucknow, U.P.), Dr Arun K Mishra (Kalputra hospital, Jaunpur, U.P.), Dr Rajiv Gupta (Madhu hospital and Lokpriya hospital, Meerut, U.P.).