Treatment for chronic hepatitis B in Brazil are funded by the Ministry of Health and by the state Departments of Health. Clinical protocol and therapeutic guidelines approve the use of adefovir, entecavir, interferon-α, lamivudine, and tenofovir for the treatment of chronic hepatitis B. The aim of this study was to establish the profile of users of these drugs in the state of Paraná. A cross-sectional study was conducted with patients under treatment in Paraná in August 2011. The following data were obtained: gender, hepatitis B used drug, International Classification of Diseases, and regional health unit. The monthly cost of these drugs for the public health system was also calculated. 1,093 patients registered were found, 70% male, and 2.6% co-infected with the delta agent. Tenofovir was the drug most commonly used (355 users). The highest prevalence was found in the regional health units of Pato Branco, Cascavel, Foz do Iguaçú, Francisco Beltrão, Toledo, Londrina, and Maringá. The annual cost for the public health system in Paraná was U$1,066,867. Through this study it was possible to investigate the distribution and profile of users of drugs for the treatment of chronic hepatitis B in Paraná in August 2011.
Currently, more than two billion people have been infected by hepatitis B virus (HBV), and roughly 350 million remain infected. Over 500,000 people die annually due to diseases related to HBV infection. In addition, the frequency of hepatitis B is still underestimated, considering that many carriers are asymptomatic. The Ministry of Health estimates that in Brazil, around 15% of the population has been exposed to the virus, and 1% have chronic disease.1–4 There is evidence of higher prevalence of HBV in urban populations with lower complexity.5 However, epidemiological studies on chronic hepatitis B in Brazil are scarce and are generally performed on specific population groups. There are high risk areas, such as Western and Southern Paraná, Western Santa Catarina, Jequitinhonha, certain regions of Mato Grosso, and Western Amazônia.2,6
The treatment of chronic hepatitis B aims to prevent or reduce the development of liver cirrhosis and hepatocellular carcinoma, and also aims to suppress viral replication, normalize ALT levels, decrease liver damage, and obtain HBsAg seroconversion.3,7 In Brazil, the public health system provides treatment for patients with chronic hepatitis B, regulated by the clinical protocol and therapeutic guidelines for the treatment of chronic viral hepatitis B and coinfection, published by the Ministry of Health.4 Pharmacological options proposed in this protocol include conventional interferon alpha (IFN-α), pegylated interferon alpha (PEG-IFN-α), and the nucleoside/nucleotide analogs adefovir dipivoxil (ADF), entecavir (ETV), lamivudine (LAM), and tenofovir disoproxil fumarate (TDF).4
The Department of Health of the state of Paraná (SESA/PR), through the Center for Drugs in Paraná (Centro de Medicamentos do Paraná – CEMEPAR), is responsible for managing the pharmaceutical care at the state level. CEMEPAR's activities are structured in the pharmaceutical care cycle: scheduling, routing the purchase, receiving, storing, and distributing to the regional health units of Paraná.
Controlling the number of registered patients, changes in doses prescribed by physicians, the switch and/or discontinuation of drugs, the influx of new patients, and the outflow of other patients (cure, noncompliance, or death) are factors of extreme importance to planning the procurement of drugs, and to the program management. Based on this demand, CEMEPAR, along with the informatics’ company CELEPAR, developed in 2004 an information system called SESAFARM, which was used as the basis for the development of the system currently employed at national level by the Ministry of Health Information System Component Specialized Pharmaceutical Services (Sistema de Medicamentos Excepcionais – SISMEDEX). This program gives public managers the record of the dispensed drugs in Paraná.
This study was carried out in order to profile the users of SISMEDEX, State of Paraná, fulfilling the provisions of the clinic protocol for the treatment of chronic viral hepatitis B co-infection.4 Variables such as the International Classification of Diseases (ICD), gender, regional health unit, drug used, and co-infection with hepatitis delta virus (HDV) were evaluated. The annual cost to the public health system of the drugs used to treat these patients in Paraná was also evaluated.
This is a cross-sectional study performed by collecting data of users registered in SISMEDEX in Paraná, during August 2011. Demographic and record dispensing data of these users were collected by selecting the search for pathology, based on the criteria established in the clinical protocol and therapeutic guidelines for the treatment of chronic viral hepatitis B and coinfection,4 considering the ICD B18.1 (without chronic hepatitis delta agent), and ICD B18.0 (chronic viral hepatitis B with delta agent). For each user the following data were collected: ICD, gender, regional health unit, and drug dispensed.
The number and percentage of patients listed were calculated for each variable. Moreover, the prevalence was calculated per 100,000 cases observed in each regional health unit. Using the chi-squared test, the observed cases were compared with the expected values for each regional health unit, in order to assess which regions had a higher or lower prevalence of chronic HBV infection than the overall prevalence of Paraná.
The drugs used in Paraná to treat chronic hepatitis B, and the number of users of each drug were also assessed.
To calculate the resources spent annually on the treatment of chronic hepatitis B in Paraná, only the costs of drugs, which were calculated according to data provided by CEMEPAR relating to remittances received from Ministry of Health in 2011, were considered. With the cost of remittances from 2011, a weighted average was calculated, which was multiplied by the number of doses per year of the drug and the number of users obtained in CEMEPAR.
In the population count conducted by the Brazilian Institute of Geography and Statistics in 2007, the population of Paraná was 10,511,862, spread over 399 municipalities.8 The number of registered users in the SISMEDEX program, receiving treatment for chronic hepatitis B during August 2011, was 1,093 (about 0.01% of the state population). Of these individuals, 328 (30%) were female and 735 (70%) were male, with significantly (p<0.05) more male patients. Coinfection with the delta agent (ICD 18.0) was found in 28 individuals (2.6%).
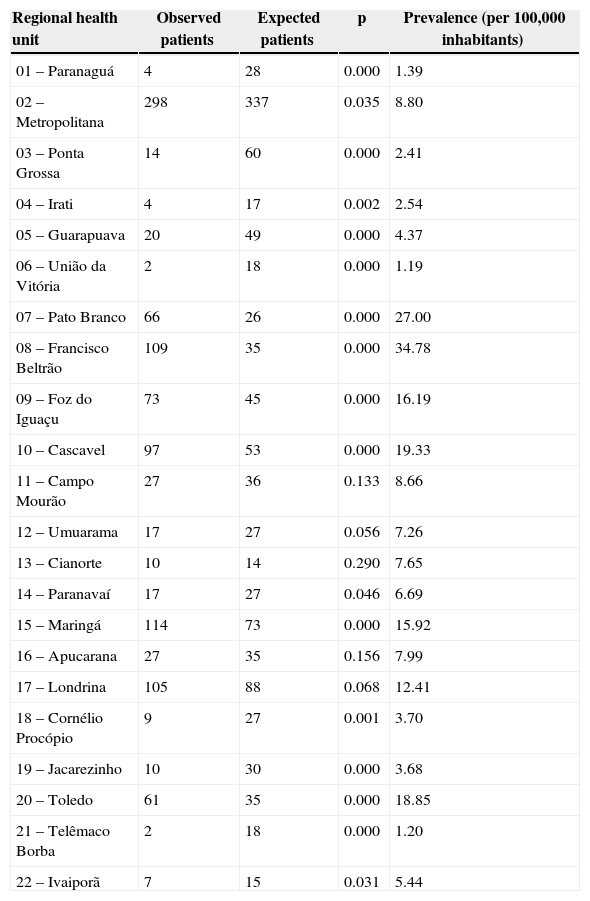
Table 1 illustrates the number of patients in each regional health unit. Using the chi-squared test, the amount expected for each region and the p-values for the data observed and expected based on population distribution we calculated. The result suggests that in most regional health units the number of patients with chronic hepatitis B was different than expected. This difference was not seen only in regions 11, 13, 16, and 17, in which the number of patients observed was similar to that expected (p>0.05). In addition, Table 1 presents the prevalence of chronic hepatitis B cases per 100,000 inhabitants.
List of observed versus expected number of registered patients with chronic hepatitis B by the regional health units of Paraná, Brazil.
| Regional health unit | Observed patients | Expected patients | p | Prevalence (per 100,000 inhabitants) |
|---|---|---|---|---|
| 01 – Paranaguá | 4 | 28 | 0.000 | 1.39 |
| 02 – Metropolitana | 298 | 337 | 0.035 | 8.80 |
| 03 – Ponta Grossa | 14 | 60 | 0.000 | 2.41 |
| 04 – Irati | 4 | 17 | 0.002 | 2.54 |
| 05 – Guarapuava | 20 | 49 | 0.000 | 4.37 |
| 06 – União da Vitória | 2 | 18 | 0.000 | 1.19 |
| 07 – Pato Branco | 66 | 26 | 0.000 | 27.00 |
| 08 – Francisco Beltrão | 109 | 35 | 0.000 | 34.78 |
| 09 – Foz do Iguaçu | 73 | 45 | 0.000 | 16.19 |
| 10 – Cascavel | 97 | 53 | 0.000 | 19.33 |
| 11 – Campo Mourão | 27 | 36 | 0.133 | 8.66 |
| 12 – Umuarama | 17 | 27 | 0.056 | 7.26 |
| 13 – Cianorte | 10 | 14 | 0.290 | 7.65 |
| 14 – Paranavaí | 17 | 27 | 0.046 | 6.69 |
| 15 – Maringá | 114 | 73 | 0.000 | 15.92 |
| 16 – Apucarana | 27 | 35 | 0.156 | 7.99 |
| 17 – Londrina | 105 | 88 | 0.068 | 12.41 |
| 18 – Cornélio Procópio | 9 | 27 | 0.001 | 3.70 |
| 19 – Jacarezinho | 10 | 30 | 0.000 | 3.68 |
| 20 – Toledo | 61 | 35 | 0.000 | 18.85 |
| 21 – Telêmaco Borba | 2 | 18 | 0.000 | 1.20 |
| 22 – Ivaiporã | 7 | 15 | 0.031 | 5.44 |
The overall prevalence of chronic hepatitis B in Paraná was 10.4 individuals per 100,000 inhabitants.
Regarding dispensed drugs, various regimens were observed. Among these, ADF, ETV, IFN-α, LAM and TDF monotherapy, and combinations such as: LAM+ADF, LAM+TDF, LAM+ETV, LAM+IFN-α, ADF+ETV, ETV+TDF, ADF+TDF, and ETV+IFN-α.
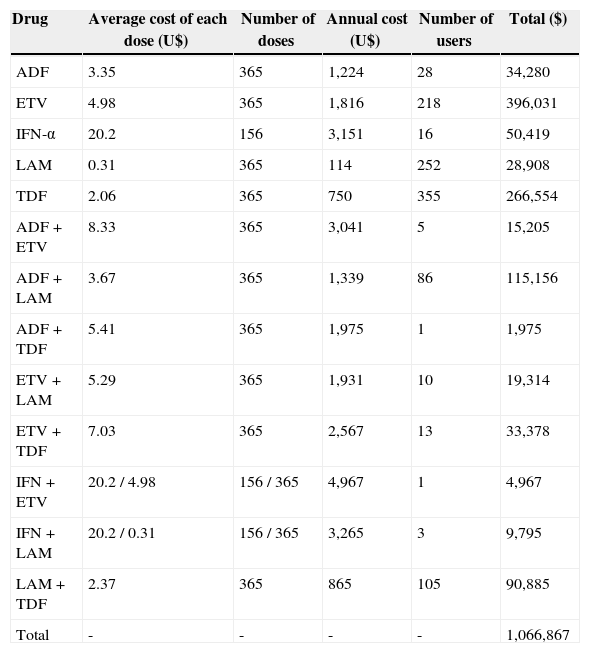
Regarding the cost of treatments, annual spending on drugs for chronic hepatitis B for the Brazilian Public Health System was USD 1,066,867, and the values are shown in Table 2.
Cost of each drug or combination for the public health system in Paraná, Brazil.
| Drug | Average cost of each dose (U$) | Number of doses | Annual cost (U$) | Number of users | Total ($) |
|---|---|---|---|---|---|
| ADF | 3.35 | 365 | 1,224 | 28 | 34,280 |
| ETV | 4.98 | 365 | 1,816 | 218 | 396,031 |
| IFN-α | 20.2 | 156 | 3,151 | 16 | 50,419 |
| LAM | 0.31 | 365 | 114 | 252 | 28,908 |
| TDF | 2.06 | 365 | 750 | 355 | 266,554 |
| ADF+ETV | 8.33 | 365 | 3,041 | 5 | 15,205 |
| ADF+LAM | 3.67 | 365 | 1,339 | 86 | 115,156 |
| ADF+TDF | 5.41 | 365 | 1,975 | 1 | 1,975 |
| ETV+LAM | 5.29 | 365 | 1,931 | 10 | 19,314 |
| ETV+TDF | 7.03 | 365 | 2,567 | 13 | 33,378 |
| IFN+ETV | 20.2 / 4.98 | 156 / 365 | 4,967 | 1 | 4,967 |
| IFN+LAM | 20.2 / 0.31 | 156 / 365 | 3,265 | 3 | 9,795 |
| LAM+TDF | 2.37 | 365 | 865 | 105 | 90,885 |
| Total | - | - | - | - | 1,066,867 |
The incidence of chronic hepatitis B varies widely, depending on geographical area, and in Brazil it is more prevalent in the states of Amazonas, Mato Grosso, Paraná, and Santa Catarina. The differences in endemicity between the various population groups of the state is not well explained, but it may be due to several factors, such as the quality and efficiency of the notifications, vaccination campaigns, basic education, sanitation, effective medical care, overcrowding dwellings, among others.2,7,9–11Although a higher incidence of hepatitis B is observed in males, there is no scientific evidence to support an increased susceptibility to viral infection in males. This result is probably due to behavioral factors. In the current study, it was found that most evaluated patients (70%) were males, which is in agreement with data found in literature.1,12
Of the 350 million chronic carriers of hepatitis B in the world, it is estimated that 18 million (approximately 5%) are coinfected with hepatitis delta virus.1,13 In this study, 2.6% of patients treated for chronic hepatitis B were found to be coinfected with delta virus.
The prevalence of chronic hepatitis B in Paraná is higher in the west and south, high risk areas for the disease.2,6 An intermediate prevalence was also observed in 2003 in the cities of Cascavel, Foz do Iguaçú, and Francisco Beltrão.14 In this study, the health regional units with the highest number of individuals on treatment (over 10 per 100,000 inhabitants) were those of Pato Branco, Cascavel, Foz do Iguacú, Francisco Beltrão, Toledo, Londrina, and Maringá.
In recent years, health expenditure has become a problem especially in developing countries. The causes of increased expenditures are diverse and may be related to the emergence of new technologies, often more effective and more expensive. Moreover, with the emergence of new diagnostic and therapeutic strategies, life expectancy has increased and consequently spending on health has increased as well. There is a constant pursuit within health systems to maximize health gains with the use of available resources. In the current study, the most commonly used drug in Paraná for the treatment of chronic hepatitis B is now TDF (355 patients). However, more resources are spent on ETV (USD 396,031/year). In this case, it is essential to conduct cost-effectiveness studies to provide the best allocation of healthcare resources.
This study made identified the varying prevalence rates of chronic hepatitis B over Paraná, according to the geographic area, suggesting that a possible difference between risk factors and protective factors may exist in each regional health unit. Moreover, the identification of the profile of users of drugs for the treatment of chronic hepatitis B in Paraná registered in SISMEDEX and cost of different treatments for the public health system can assist in determining strategies for management and prevention of morbidity and mortality.
Conflict of interestAll authors declare to have no conflict of interest.