A nosocomial polyclonal outbreak associated to bacteremia caused by different Burkholderia cepacia complex (BCC) species and clones is reported. Molecular characterization identified Burkholderia stabilis, Burkholderia contaminans, and Burkholderia ambifaria among BCC isolates obtained from patients in neonatal and adult intensive care units. BCC was also isolated from an intrinsically contaminated ultrasound gel, which constituted the presumptive BCC source. Prior BCC outbreak related to contaminated ultrasound gels have been described in the setting of transrectal prostate biopsy. Outbreak caused strains and/or clones of BCC have been reported, probably because BCC are commonly found in the natural environment; most BCC species are biofilm producers, and different species may contaminate an environmental source. The finding of multiple species or clones during the analysis of nosocomial BCC cases might not be enough to reject an outbreak from a common source.
The Burkholderia cepacia complex (BCC) encompasses at least 17 related Gram-Negative bacilli species as judged by different phenotypic and genotypic analyses.1 BCC members can cause infections in cystic fibrosis, chronic granulomatous disease, and hospitalized patients.2 BCC members are among the most frequent sources of nosocomial outbreaks due to intrinsically contaminated substances other than blood products.2 Here, we describe an outbreak of bacteremia caused by BCC strains between April and July 2013. Subject's clinical charts were reviewed and microbiological testing of substances and solutions representing potential sources of the outbreak was performed. Eighty samples from different wards and commercial products commonly used in the Neonatal Unit (NU) and Intensive Care Unit (ICU) were tested for BCC presence using selective culture media (Burkholderia agar, BioMerieux Inc, Mercy L¿etoil, France). All suspected isolates were phenotypically identified as belonging to the BCC by oxidase, OF-glucose, sculin hydrolysis, lysine decarboxylation, and DNA hydrolysis tests, as well as by the semi-automatized API-20NE (BioMerieux Inc, Mercy L¿etoil, France) method. Regrettably, only nine BCC isolates were available for molecular analysis (six from blood cultures and three from ultrasound gels). These strains were identified to the species level by recA gene sequence comparisons3 and analyzed for genomic relatedness by both degenerate oligonucleotide-primed PCR4 and repetitive extragenic palindromic-PCR.5
The outbreak involved 11 patients with 17 BCC isolates recovered from blood cultures; seven of these 11 subjects were hospitalized in the NU, all of them were preterm neonates with respiratory distress, three other patients were in the ICU, two of which had recent cardiovascular surgery, and one patient was in the General Ward. The mean (range) time of hospitalization of these patients until the development the bacteremia was 5.55 (0–15) days; one neonate developed BCC bacteremia the date of birth, probably reflecting horizontal transmission. In seven patients, BCC strains were recovered only from baseline blood cultures; two more patients had positive surveillance blood cultures on day 2, and two other patients had bacteremia also in a 3rd set of blood cultures. Three of the 11 patients died (two adults and one neonate) during the hospitalization, although none of these deaths were attributed to the BCC bacteremic episode. It was noted that the seven neonates and the four adult patients underwent a mean (range) of 5 (1–10) ultrasounds, including transthoracic and transfontanellar ones, and 2 (1–3), respectively.
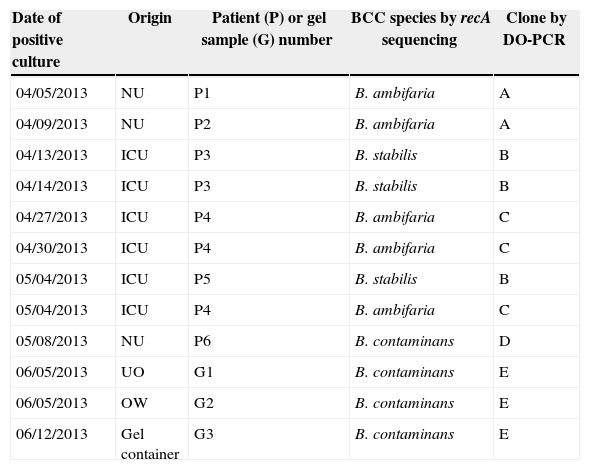
Eighty environmental samples were taken for culture, including several solutions of antiseptics (iodopovidone, hydrogen peroxide, cholorhexidine, and alcohol-gel), drugs (fentanyl citrate, morphine, tobramycin drops), multiple surfaces in the surgical room, ICU and NU, and other commonly used materials such as gels for ultrasound, liquid soap, and vaseline; BCC strains were isolated only from ultrasound scanning gels (Table 1). A quantitative culture done from an unopened 5-L container ultrasound gel displayed growth of 4.66log10CFU/mL (mean of two samples) of BCC cells. Molecular analyses based on recA gene sequence of nine isolates obtained from six patients in NU and ICU wards indicated the presence of three different species of the BCC complex (Table 1): Burkholderia ambifaria was identified in patients 1 and 2 (NU) and patient 4 (ICU), Burkholderia stabilis in patient 3 and 5 (ICU), and Burkholderia contaminans in patient 6 (NU). All BCC isolates were susceptible to ceftazidime, meropenem, minocycline, and trimethoprim-sulfamethoxazole by disk diffusion methods.
Description of the 12 Burkholderia cepacia complex strains isolated from blood cultures and from ultrasound lubricant gel.
| Date of positive culture | Origin | Patient (P) or gel sample (G) number | BCC species by recA sequencing | Clone by DO-PCR |
|---|---|---|---|---|
| 04/05/2013 | NU | P1 | B. ambifaria | A |
| 04/09/2013 | NU | P2 | B. ambifaria | A |
| 04/13/2013 | ICU | P3 | B. stabilis | B |
| 04/14/2013 | ICU | P3 | B. stabilis | B |
| 04/27/2013 | ICU | P4 | B. ambifaria | C |
| 04/30/2013 | ICU | P4 | B. ambifaria | C |
| 05/04/2013 | ICU | P5 | B. stabilis | B |
| 05/04/2013 | ICU | P4 | B. ambifaria | C |
| 05/08/2013 | NU | P6 | B. contaminans | D |
| 06/05/2013 | UO | G1 | B. contaminans | E |
| 06/05/2013 | OW | G2 | B. contaminans | E |
| 06/12/2013 | Gel container | G3 | B. contaminans | E |
NU, Neonatal Unit; ICU, Intensive Care Unit; UO, Ultrasound Office; OW, Obstetric Ward, DO-PCR, degenerate oligonucleotide-PCR.
Among the clinical isolates, genotypic characterization revealed two different clones of B. ambifaria and a single clone of both, B. stabilis and B. contaminans (Table 1). Two different BCC species coexisted in the NU (B. ambifaria and B. contaminans) and the ICU (B. stabilis and B. ambifaria). In addition, two different B. ambifaria clones were detected, both from different wards (Table 1). B. contaminans was isolated in different samples of ultrasound gel as it was from patient 6 although this isolate was a different clone (Table 1). Repetitive extragenic palindromic-PCR confirmed the clonal distinctness of the BCC isolates analyzed above (data not shown). These results support the polyclonal outbreak of BCC strains caused by multiple species (B. ambifaria, B. stabilis, and B. contaminans) and clones (e.g. B. ambifaria, B. contaminans).
Interesting, BCC members can hydrolyze parabens, which are p-hydroxybenzoic acid esters with antimicrobial properties commonly added as stabilizers to ultrasound gels6; BCC strains can therefore survive and proliferate in these gels.6 Even though the sterility of substances in contact with intact skin such as ultrasound gels is generally not required, the US Food and Drug Administration had to recall commercial ultrasound gels contaminated with Pseudomonas aeruginosa and Klebsiella oxytoca, recommending the use of sterile ultrasound gel for invasive procedures, leaving the use of non-sterile (open) containers for procedures performed on intact skin and for low risk patients.7 Of note, BCC invasive infections related to contaminated ultrasound gels have been only described in the setting of transrectal prostate biopsy.6,8 Current report represents the third outbreak of BCC presumably associated to ultrasound gel. We speculate that the invasive procedures done in neonate hosts and patients undergoing cardiovascular surgery might have predisposed them to develop bacteremia after significant BCC skin colonization from contaminated gel.
The striking feature of this outbreak is the presence of multiple BCC species and clones since most BCC outbreaks have been associated to a single clone. However, outbreaks caused by different strains and/or clones have been reported,9–11 including BCC contamination of hospital water,9 intravenous bromopride vials,10 and non-identified environmental sources.11 Since BCC bacteria are commonly found in the natural environment and most BCC species are biofilm producers, different species may contaminate an environmental source (as it has been described in cystic fibrosis patients12), eventually leading to a polyclonal nosocomial outbreak. Unfortunately, we could not confirm this hypothesis as we only recovered one B. contaminans clone from the ultrasound gel samples.
In summary, the sudden appearance of BCC invasive cases, the isolation of BCC from ultrasound gels including an unopened container, and the abrupt interruption of new cases after removal of ultrasound gel stocks led us to speculate that this substance might have been the source of the nosocomial BCC outbreak. The finding of multiple species or clones during the analysis of nosocomial BCC cases might not be enough to reject an outbreak from a common source.
Conflicts of interestThe authors declare no conflicts of interest.
We are indebted to Dr. Alejandro M. Viale for critical reading of the manuscript.
This work was partially supported by grants to ASL from the Ministerio de Salud, Provincia de Santa Fe and Secretaría de Ciencia y Técnica, Universidad Nacional de Rosario.