Data on the burden of disease and circulation patterns of influenza B lineages for Brazil are limited. This review aims to describe the pattern of influenza B occurrence in Brazil to have a better understanding of its epidemiology and its relevance when considering seasonal influenza vaccine composition.
A review of the data including analysis of international and local surveillance data as well as information from online search of databases using Medical Subject Headings terms in conjunction with screening of abstracts from scientific events was performed.
Based on international epidemiologic surveillance data, moderate levels of influenza B disease (19%; 2006–2014) were observed. Of these nine years, it was possible to compare data from three years (2007, 2008 and 2013) which have information on the circulating influenza B lineage. Co-circulation of influenza B lineages was observed in all these three influenza seasons, of which, during one season, a high degree of mismatch between the vaccine lineage and the predominant circulating lineage (91.4% [2013]) was observed. Local surveillance data reveal a distinct and dynamic distribution of respiratory viruses over the years. Data from published literature and abstracts show that influenza B is a significant cause of disease with an unpredictable circulation pattern and showing trends indicating reemergence of the B/Victoria lineage. The abstracts report notable levels of co-circulation of both influenza B lineages (2000–2013). Mismatch between the Southern hemisphere vaccine and the most prevalent circulating viruses in Brazil were observed in five influenza seasons.
The evidence on co-circulation of two influenza B lineages and mismatched seasons in Brazil indicates the benefit of quadrivalent influenza vaccines in conferring broader seasonal influenza protection. Additionally, improving influenza surveillance platforms in Brazil is important for monitoring disease trends and the impact of introducing seasonal influenza vaccination.
Influenza is a highly infectious acute viral illness resulting in significant morbidity as well as healthcare resource utilization. In healthy individuals influenza is generally self-limiting, but can often cause complications.1,2 There are 3 types of seasonal influenza viruses – A, B, and C. Influenza A causes moderate to severe illness and affects individuals of all age groups. Influenza B can cause disease of similar severity as influenza A, and even though the morbidity is higher in children, all age groups can be affected.3,4 The influenza B virus is more stable than influenza A, with less antigenic drift and consequent immunologic stability, and does not undergo the process of antigenic shift. Influenza C is rarely reported as a cause of human illness, probably because most cases are subclinical.5 Both influenza A and B cause annual epidemics worldwide and are estimated to result in 3–5 million cases of severe illness, and 250,000–500,000 deaths.6
Influenza vaccination is the most important prophylactic intervention against infection. Until the 2012–2013 influenza season, use of trivalent inactivated influenza vaccines, containing two influenza A strains (A[H1N1] and A[H3N2]) and one influenza B lineage (B/Yamagata or B/Victoria) was recommended for use in immunization programs by the World Health Organization (WHO).6 As influenza viruses undergo frequent changes in their surface antigens, new influenza vaccines are designed annually to match the circulating virus subtype expected for the next influenza season.7 The selection of the influenza B lineage is considered critical in determining the effectiveness of vaccination programs.7 Unfortunately, the correct prediction of the predominating circulating B lineage is quite difficult, often leading to inaccuracies in prediction, causing a mismatch between the recommended vaccine lineage and the circulating influenza B lineage. Prior studies have raised concerns that mismatches can result in lower vaccine effectiveness, due to the absence of cross-protection between antigenically distinct influenza B lineages, leading to more influenza cases,8,9 and an increase in influenza-related medical resource utilization and costs.2,10 In addition to this, Matias et al.11 have found that influenza B-associated mortality could serve as a surrogate marker of disease severity.
In the Latin America and Caribbean region, seasonal influenza causes high morbidity placing a substantial economic burden on healthcare systems and society.12 Data on the burden of influenza disease for Brazil are limited, most likely due to underreporting.12 Between 2000 and 2008, data from the influenza surveillance system in Brazil revealed that influenza-like illness (ILI) led to a total of 4.39–16.92% of hospital consultations, and in 2008, of all positive reported influenza cases, 43.29% (95% CI: 37.59–49.13) were influenza B.12
The Ministry of Health (MoH, “Ministério da Saúde”) of Brazil promotes annual national influenza vaccination campaigns. Over the years, there has been a gradual expansion of the recommended groups for annual influenza immunization in Brazil.13 Since 1999, influenza vaccination was introduced for elderly people aged above 65 years and other groups vulnerable to complications (patients with co-morbidities). In the year 2000, individuals 60 years or older were included for vaccination.14,15 During 2011–2012, in addition to elderly people, vaccination was extended to children aged six months to those aged below two years, pregnant women, healthcare professionals, and indigenous people. In 2013, women after child birth, individuals with chronic disease and transplant, and individuals in detention facilities were included for annual influenza vaccination. In 2014, children aged 2–4 years were also included in the recommended target at-risk groups for vaccination. The information system of National Immunization Program for Brazil (“Programa Nacional de Imunização”)13 reported that across all target vaccination groups, overall mean vaccination coverage of 86.8% was reached in 2014.13 During all influenza vaccination campaigns in Brazil, trivalent vaccines were used according to WHO recommended vaccine composition for South hemisphere.16
Although high vaccination coverage levels have been reached in these target vaccination groups, little is known about the effectiveness of vaccination programs in Brazil.14,17 A number of factors, in particular vaccine coverage, is known to influence the effectiveness of influenza vaccination programs. However, the Brazilian MoH data shows vaccine coverage to be high in almost all years since the introduction of vaccination. Importantly, the extent to which the vaccine recommended influenza B virus lineage matches the influenza virus lineage circulating in the population during an influenza season is known to impact the effectiveness of seasonal influenza vaccination programs.14 Data on laboratory surveillance of the influenza B virus in Brazil are limited, specifically data on the burden of disease and circulation patterns of influenza B lineages. The present integrative review of publicly available data aims to consolidate findings on the pattern of influenza B occurrence in Brazil to have a better understanding of influenza B epidemiology and its relevance to seasonal vaccine composition.
Material and methodsInformation sources and search strategyEpidemiological surveillance systemsDifferent sources were used to retrieve information on epidemiological surveillance. We referred to international data sources to check WHO recommendations on the vaccine composition in the Southern hemisphere,18 and information on circulating influenza lineages for Brazil, the South America region and globally from the WHO/FluNet database which provides data through its network – Global Influenza Surveillance and Response System (GISRS) laboratories.19 Consolidation of available national epidemiological information on Influenza in the Epidemiological Bulletins of the Brazilian MoH was also performed. Bulletins available from 2009 to Epidemiological Week 35 of 2014 were considered.15 In Brazil, two types of surveillance exist: Sentinel surveillance of ILI and Universal Severe Acute Respiratory Syndrome (SARS) surveillance. ILI is defined as fever followed by cough or sore throat and symptoms onset within the last seven days. SARS is defined as fever followed by cough or sore throat and experiencing dyspnea, requiring hospitalization. Oxygen saturation levels lower than 95%, respiratory distress, or respiratory rate increase, are also considered in SARS. The sentinel ILI surveillance, which has a network of units distributed throughout the country's geographic regions, is used in the identification and characterization of circulating respiratory viruses in viral isolations. The universal SARS surveillance, which was implemented post-identification of the influenza A(H1N1) 2009 pandemic strain (pdm09), monitors hospitalized cases and deaths. In both systems, data are collected by means of standardized forms and entered into the online information systems: Influenza epidemiological surveillance system called SIVEP-Gripe for ILI cases, and the National Information System for Notifiable Diseases known as SINAN Influenza Web for SARS cases. Results from tests performed at the 27 Public Health Central Laboratories (“Laboratórios Centrais de Saúde Pública, Lacen”) are routinely included in these systems. The diagnostic kits currently available identify the influenza A(H1N1)pdm09 viral strain (determined by WHO), influenza A(H3N2), influenza A not subtyped, and influenza B virus. The antigenic characterization of the circulating influenza virus lineage is performed by three laboratories in Brazil (“Instituto Evandro Chagas” – Pará, “Instituto Adolfo Lutz”, São Paulo and “Instituto Oswaldo Cruz”, Rio de Janeiro). These laboratories are part of a network of 140 National Influenza Centers acknowledged by WHO as members of the GISRS.
Online literature search and review of abstractsAn integrative literature review was performed using online database tools such as PubMed/MEDLINE, Lilacs and Scielo. Searches on online databases were conducted using the following search strategy built for the PubMed database (via Medical Subject Headings [MeSH] controlled vocabulary) and adjusted for other databases according to their specificities: “(((((influenza [Title/Abstract]) OR influenzae)) OR ((orthomyxoviridae [MeSH Major Topic]) OR influenza, human [MeSH Major Topic]))) AND Brazil”. Studies on influenza B, with Brazil as the place of study and those with virological influenza B information were considered. Articles published between 2007 and 2014 were considered and a language constraint was not applied. Articles and abstracts with general influenza reports or without subtyping (A or B) were not included. One reviewer screened titles and abstracts for relevance, as the defined inclusion criteria were restrictive. An additional research was conducted in abstracts of the Annals of Scientific Events related to the area of research. A total of 10 events were considered.20–29 All authors agreed with the selected publications.
AnalysesData on viral lineage isolation and characterization, vaccination campaign, viral lineage surveillance and strain match-mismatch from different sources were collected and consolidated. Statistical analysis was not performed, and thus all data are descriptive.
ResultsInfluenza viral strain dataEpidemiologic surveillance systemsInformation on influenza A and B subtype patterns were obtained from the WHO/FluNet database (up to epidemiological week 35, 2014). Globally and for South America, 1,122,415 and 95,040 samples were recorded in the database (WHO/FluNet-GISRS), respectively; of these 45,856 and 937 samples were typed as influenza B. In South America, of 937 influenza B samples, only 279 were subtyped, of which 252 (90.3%) samples were of the B/Yamagata lineage and 27 (9.7%) were subtyped as the B/Victoria lineage. In comparison, the WHO/FluNet database recorded 7756 samples from Brazil of which 100 samples were positive for the influenza B virus. Among these no influenza B sample was subtyped.
Epidemiologic surveillance data showed that the influenza B disease burden was moderate in both South America and Brazil. Based on WHO/FluNet (September 07, 2014), in Brazil, between 2006 and 2014, influenza B circulated in all the years, with an average of 19% of all circulating influenza viruses (A and B), varying from 1.0% to 42.6% (2006: 21.8%; 2007: 27.5%; 2008: 42.6%; 2009: 1.0%; 2010: 14.0%; 2011: 15.5%; 2012: 8.9%; 2013: 30.6% and 2014: 10.2%). In South America, between 2006 and 2014, a similar trend was observed. Influenza B virus circulated in all the years, with an average 16% of influenza B detected in relation to all of the circulating influenza viruses (A and B), varying from 0.5% to 30.1% (2006: 14.0%; 2007: 12.6%; 2008: 30.1%; 2009: 0.5%; 2010: 16.9%; 2011: 4.2%; 2012: 28.3%; 2013: 19.6% and 2014: 14.0%).
Trends on the etiological distribution of influenza disease were studied from the Epidemiological Bulletins of the Brazilian MoH based on two types of surveillance systems. Data from the Sentinel Surveillance of ILI (2009–2014) shows a dynamic pattern of distribution of different respiratory viruses in Brazil through the years, by epidemiological week, with predominance of influenza and respiratory syncytial virus (RSV). Influenza virus circulation demonstrates a seasonal pattern with highest occurrence from April to June, while RSV circulation showed more intense activity in the first months of the years. Influenza B follows similar seasonal trends; however it seems to occur slightly later than influenza A. During the seasons of 2009, 2010, 2011 and 2012, influenza A and RSV predominated followed by influenza B. In the year 2013, the absolute numbers of influenza B typed samples in ILI cases was higher (n=727; 36.2% [2013]) compared to the previous years (n=128; 21.0% [2009], n=213; 46.3% [2010], n=191; 34.5% [2011], n=not available [2012]) and 2014 (n=150; 12.7% [2014, up to epidemiological week 35]). There were regional differences in the seasonal patterns of influenza virus, however strain specific information is lacking.
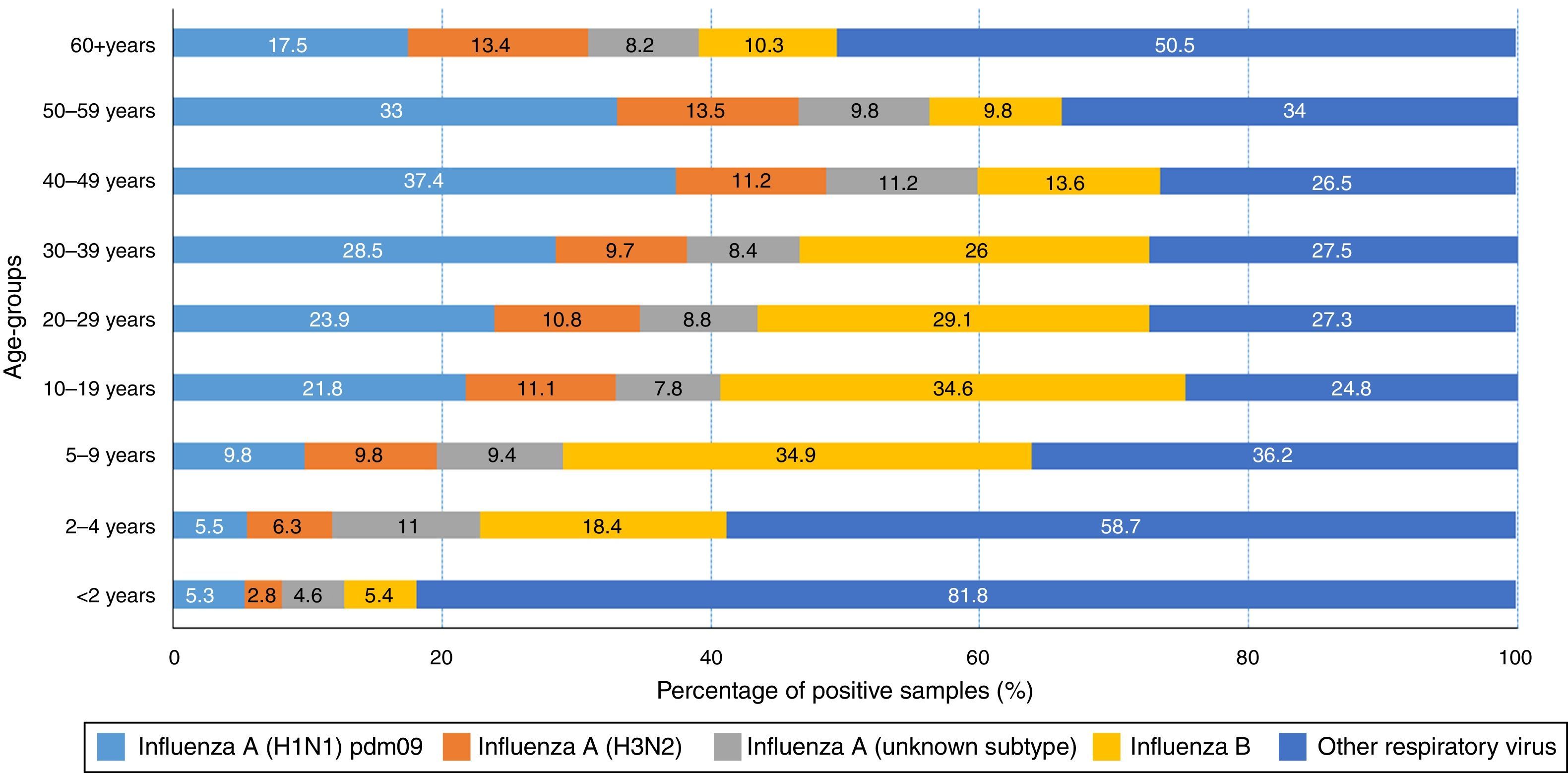
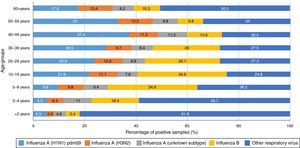
Data on virus circulation by age group (2013, Fig. 1) shows that influenza A(H1N1)pdm09 virus was predominant in individuals aged 30–59 years, especially among individuals aged 40–49 years of age (37.4%). On the other hand, influenza B virus was predominant in younger people, aged 5–29 years, with highest proportions observed in children aged 5–9 years and 10–19 years (34.9% and 34.6%, respectively).
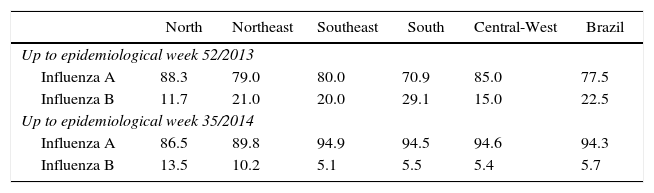
The pattern of hospitalized SARS cases from the Universal SARS surveillance showed that in 2013, although influenza A(H1N1)pdm09 was predominant, 22.5% of SARS cases positive for influenza (1337/5935, with 85 deaths) were caused by the influenza B virus. Regional variation was observed between the regions – South region (29%) and North region (12%). In 2014 (up to epidemiological week 35) 6% of SARS cases positive for influenza (75/1307, with 9 deaths) were caused by the influenza B virus (Table 1).
Percentage of SARS cases positive for influenza according to identified virus type by region of residence and year, Brazil.
| North | Northeast | Southeast | South | Central-West | Brazil | |
|---|---|---|---|---|---|---|
| Up to epidemiological week 52/2013 | ||||||
| Influenza A | 88.3 | 79.0 | 80.0 | 70.9 | 85.0 | 77.5 |
| Influenza B | 11.7 | 21.0 | 20.0 | 29.1 | 15.0 | 22.5 |
| Up to epidemiological week 35/2014 | ||||||
| Influenza A | 86.5 | 89.8 | 94.9 | 94.5 | 94.6 | 94.3 |
| Influenza B | 13.5 | 10.2 | 5.1 | 5.5 | 5.4 | 5.7 |
Note: Data obtained from Sinan Influenza Web/SVS/MS; SARS, Severe Acute Respiratory Syndrome.
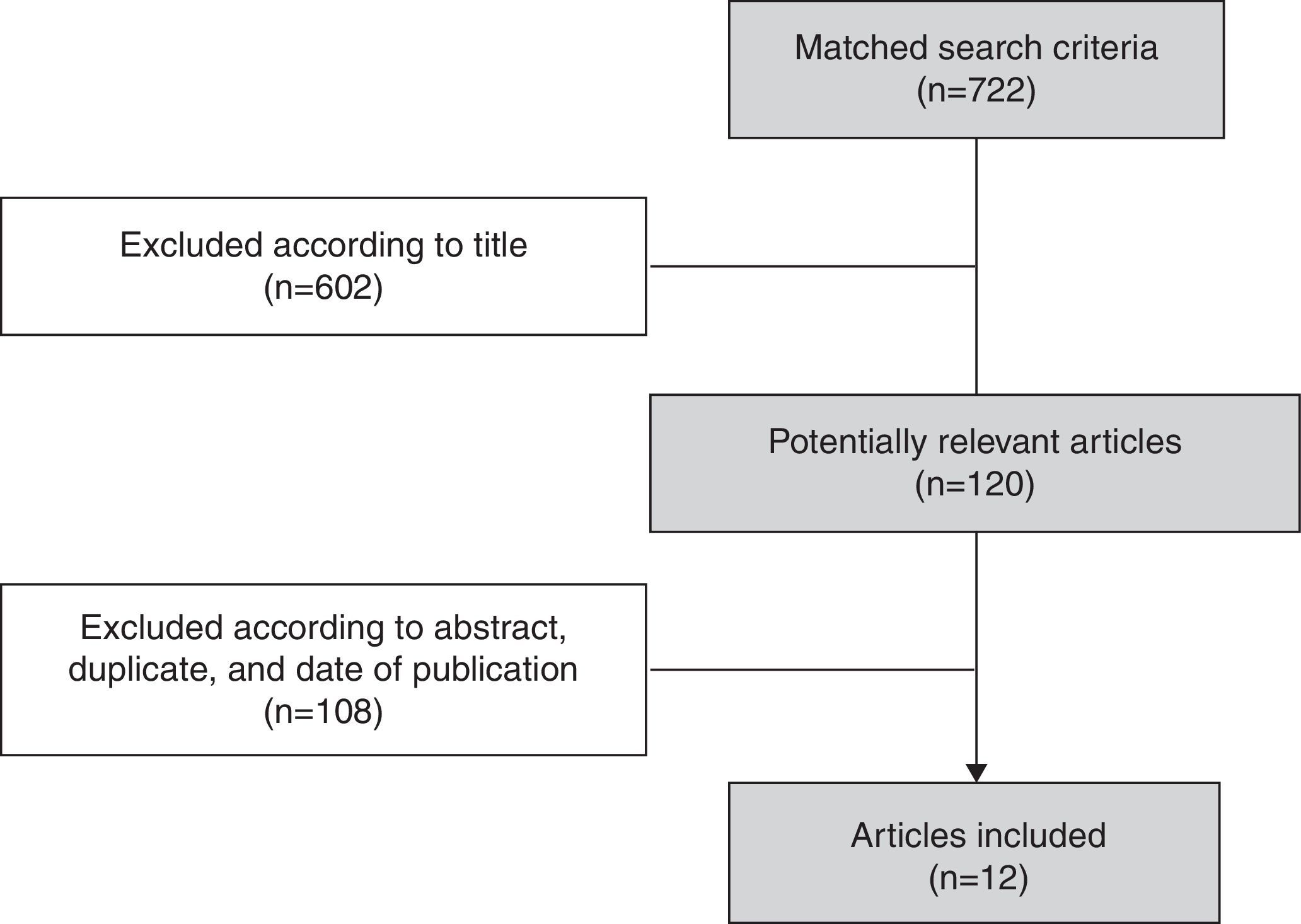
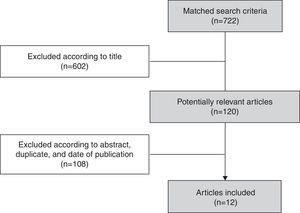
A total of 722 articles were identified, of which 602 articles were excluded after the review of titles as they were related to influenza other than influenza B. Subsequent to the screening of titles, abstracts of the 120 potentially relevant articles were reviewed and, as a result, 108 articles were excluded when the eligibility criteria were not met (Fig. 2). In all, 12 articles presenting evidence on influenza B virus circulation in Brazil for the years 1996–2013 were included.30–41 Details for each of the studies are shown in Supplementary File 1.
Two studies detailed characteristics of an influenza outbreak in February 2012 on cruise ships.32,35 Fernandes et al.35 reported findings from the first influenza outbreak detected by Brazilian public health authorities in a vessel cruising in South America. Of 11 hospitalized cases of acute respiratory illness, there were six cases with influenza B virus detected in the nasopharyngeal isolates.35 In another study by Borborema et al.32, it was shown that the influenza B virus was the cause of the outbreak (detected in seven individuals with respiratory illness).
Other reports from the online literature review were primarily from retrospective descriptive studies. Freitas36 showed that influenza B was attributable to moderate levels of disease during 2000 and 2010 (3–17%), and its occurrence was notable in the South and Central-West regions. Paiva et al.37 confirmed the reemergence of the B/Victoria viruses in São Paulo, Brazil, during the years 1996–2012.
Review of abstractsA total of three abstracts from Annals of Scientific Events, in their periodic issues from 2007 to 2014 were selected42–44 (Supplementary File 1). Perosa and Bellei44 studied influenza B circulation patterns during 12 seasons (2001–2013) for São Paulo city and identified that of 96 samples subtyped in patients (mean age: 23.9 years), the majority of samples had the B/Victoria lineage (58.3%) and the remaining 41.7% belonged to the B/Yamagata lineage.44 Oliveira et al.42 studied mismatch between vaccine and circulating influenza lineages in different regions of Brazil between 2001 and 2013 and observed varying levels of co-circulation (up to 100%) of influenza B lineages.42 A study by Paiva et al.43 showed that across different regions of Brazil, 16% of influenza in clinical specimens from sentinel units in 2013 were caused by the B/Victoria lineage while the remaining cases were caused by the influenza A virus.
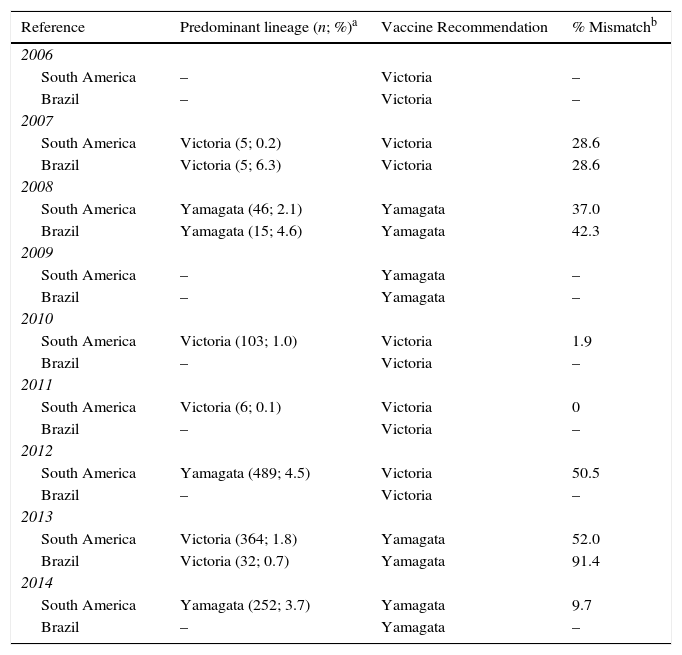
Influenza B lineage match-mismatch in BrazilEpidemiologic surveillance systemsIn Table 2, based on WHO/FluNet reports, consolidated annual data on the lineages that were part of the vaccine, the proportion of predominant circulating lineages and the proportion of lineage mismatch is shown for South America and Brazil. Data for Brazil was available for nine years; of these, it was only possible to compare data from three years (2007, 2008 and 2013) which have information available on the circulating influenza B virus lineage. In Brazil, co-circulation of both influenza B lineages was detected in all these three influenza seasons, and a high level of mismatch (91.4%) was observed in one year (2013) (Table 2). In the same year for South America 52.0% (2013) of mismatch was observed.
Comparison of influenza virus vaccine lineage and circulating lineage in the population, South America and Brazil, 2006–2014 (WHO/FluNet).
| Reference | Predominant lineage (n; %)a | Vaccine Recommendation | % Mismatchb |
|---|---|---|---|
| 2006 | |||
| South America | – | Victoria | – |
| Brazil | – | Victoria | – |
| 2007 | |||
| South America | Victoria (5; 0.2) | Victoria | 28.6 |
| Brazil | Victoria (5; 6.3) | Victoria | 28.6 |
| 2008 | |||
| South America | Yamagata (46; 2.1) | Yamagata | 37.0 |
| Brazil | Yamagata (15; 4.6) | Yamagata | 42.3 |
| 2009 | |||
| South America | – | Yamagata | – |
| Brazil | – | Yamagata | – |
| 2010 | |||
| South America | Victoria (103; 1.0) | Victoria | 1.9 |
| Brazil | – | Victoria | – |
| 2011 | |||
| South America | Victoria (6; 0.1) | Victoria | 0 |
| Brazil | – | Victoria | – |
| 2012 | |||
| South America | Yamagata (489; 4.5) | Victoria | 50.5 |
| Brazil | – | Victoria | – |
| 2013 | |||
| South America | Victoria (364; 1.8) | Yamagata | 52.0 |
| Brazil | Victoria (32; 0.7) | Yamagata | 91.4 |
| 2014 | |||
| South America | Yamagata (252; 3.7) | Yamagata | 9.7 |
| Brazil | – | Yamagata | – |
Note: Data taken from WHO/FluNet (07 September, 2014).19 “–” indicates that data is not available for this year.
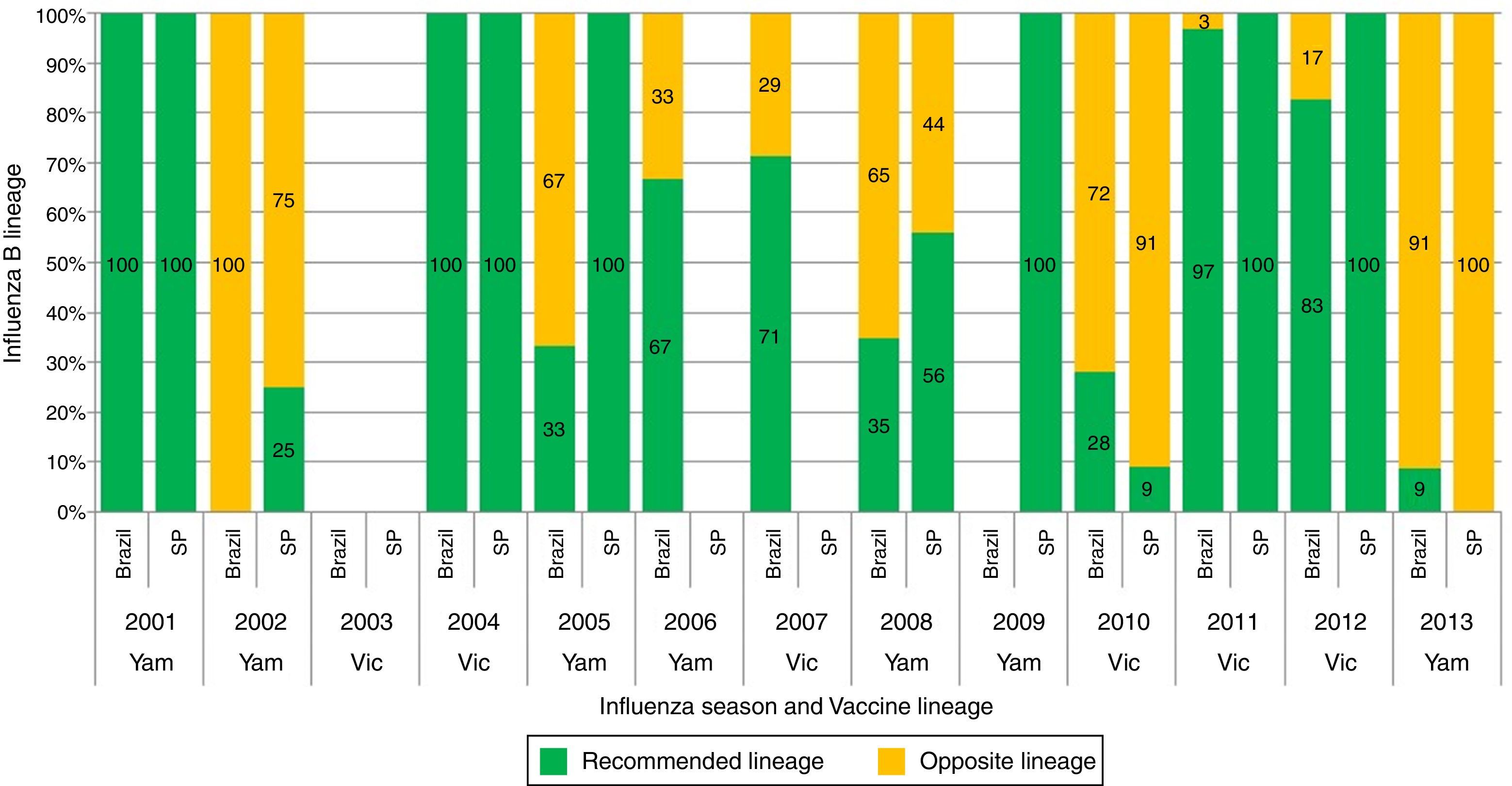
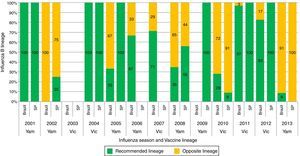
None of the articles from the online literature search in particular studied influenza B lineage match-mismatch in Brazil. However, from the reviewed abstracts, all three focused on B lineage match-mismatch in Brazil.42–44 Overall, mismatch levels in the range 0–100% were observed between the circulating influenza B virus lineage and the vaccine recommended lineage in Brazil and São Paulo city between 2000 and 2013 (Oliveira et al.42 and Perosa and Bellei44) (Fig. 3). Oliveira et al.42 showed that the B/Victoria and B/Yamagata lineages have co-circulated in Brazil since 2005. Key mismatches between the WHO recommended Southern hemisphere vaccine composition and the most prevalent circulating viruses in Brazil were observed in the years 2002 (100%), 2005 (67%), 2008 (65%), 2010 (72%) and 2013 (91%).42 A separate dataset on influenza B circulation from the São Paulo city also showed a high degree of mismatch in three seasons i.e. years 2002 (75%), 2010 (91%) and 2013 (100%)44 (Fig. 3). Moderate levels of regional variation across Brazil were evident (data not shown). Paiva et al.43 reported a complete mismatch (100%) between the circulating influenza B virus lineage and the vaccine recommended lineage in the 2013 influenza season in different regions of Brazil.
Circulation of Influenza B lineages according to season (year) and recommended vaccine lineage, Brazil and São Paulo city, 2001–2013. Note: Data taken (with permission) for Brazil from Oliveira et al.42 and for São Paulo city from Perosa and Bellei44; Samples for Brazil are estimated from regional data; Samples for the South (Rio Grande do Sul, Parana, Santa Catarina states), Southeast (Minas Gerais, Espirito Santo, Rio de Janeiro states) and Northeast (Bahia, Alagoas, Sergipe states) for which the samples were sequenced at WHO/National Influenza Center, Rio de Janeiro; Samples from other states were downloaded from the database of The Global Initiative on Sharing All Influenza Data; SP, São Paulo; Vic, B/Victoria; Yam, B/Yamagata.
Data on influenza B disease burden for Brazil are limited, especially with regards to influenza B disease burden and subtypes, which are important when considering the use of influenza vaccines. In this review, we aimed to summarize the available evidence base on influenza B disease patterns in Brazil in the international and local public health databases, as well from the literature and plenary scientific events. The review of these data highlights important patterns of influenza B circulation in Brazil. Based on WHO/FluNet reports, moderate levels of influenza B burden (1.0–42.6%) were observed over nine years, with the exception of 2009 (1.0%), which could potentially have been due to the displacement of influenza B circulation resulting from the influenza A pandemic in 2009. This finding indicates the unpredictability of influenza B circulation.
Based on WHO/FluNet reports, it was possible to compare data from only three years during 2006–2014 (2007, 2008 and 2013) which have information on the circulating influenza B virus lineages. Significant levels of co-circulation of both influenza B virus lineages during the three influenza seasons between 2006 and 2014 were observed. These data indicate that in 2013, a high degree of mismatch between the vaccine and the predominating circulating lineage (91.4%) occurred, and during the other two influenza seasons, a partial mismatch was reported.
The three reviewed abstracts, which specifically report findings on influenza B mismatch, corroborate this unpredictable behavior of influenza B disease in Brazil for many other seasons for which data were not available in the International Epidemiological surveillance data. Significant levels of co-circulation of both influenza B lineages (B/Victoria and B/Yamagata) and lineage mismatch between the vaccine and circulating lineage was reported for Brazil in five influenza seasons (2002, 2005, 2008, 2010, and 2013).42–44 Previous studies suggest that even with a partial mismatch over the years, due to the unpredictability of influenza B lineage circulation, the disease burden (in terms of clinical cases, hospitalizations and health care resource utilization) and societal burden can be considerable.2,9 For example, a mismatch in the 2007–2008 influenza season in the United States was estimated to have costed health care providers and society well over $100 million and $1 billion, respectively.45 In another study conducted in Taiwan, it was reported that an epidemic predominated by influenza B/Yamagata lineage occurred during the 2011–2012 season during which the trivalent influenza vaccine contained influenza B/Victoria lineage. Expectedly, the morbidity and mortality of this vaccine mismatched epidemic was substantial (influenza B: 60.7% and 66.9% of confirmed influenza cases and deaths, respectively; 87.2% of samples showed the presence of vaccine opposite lineage).46
Surveillance data on the age distribution of influenza cases is available only for 2013. This data showed that influenza B virus was predominantly observed in younger individuals aged 5–29 years, with the highest proportions in children aged 5–9 years and 10–19 years. Even though this observation was restricted to only one year with 90% mismatch it is in line with previous reports wherein influenza B has been reported to affect more schoolchildren and the appearance of the new B lineage reinforced this pattern.4 An implication of this finding is that children and adolescents might benefit the most from the addition of a second influenza B lineage to the seasonal influenza vaccine. Moreover, it could be useful for the entire population as it is widely considered that vaccinating children can reduce influenza illness in family members and other susceptible populations in the community by reducing the risk of exposure and subsequent influenza infection and related complications.47 A similar finding by Heikkinen et al.10 reports a substantial population-level impact of outbreaks resulting from mismatched seasons in Finland with this impact predominating in children and adolescents.
From the analysis of the Epidemiological Bulletins of the Brazilian MoH based on two types of surveillance systems in Brazil, it is shown that the proportions of ILI and SARS cases positive for influenza B were moderate. It was observed that despite high levels of vaccination coverage (91.6%) observed across all target groups in 2013,13 the proportion of registered SARS cases due to influenza B in 2013 was 22.5% (1337/5935; Universal SARS surveillance), a year in which 91.4% of the influenza B laboratory samples did not correspond to the lineage contained in the trivalent vaccine (WHO/FluNet). In the same year, 85 deaths due to influenza B were registered. This occurrence could have been due to an outbreak of SARS caused by influenza B. It was demonstrated by Matias et al.11 that influenza B-associated mortality could potentially be a valid indication of disease severity. Thus the evidence presented here for one year underscores the importance of a universal influenza vaccination strategy compared to targeted immunization as universal immunization at high vaccine coverage levels could potentially interrupt transmission reducing the occurrence of outbreaks and severe disease.
Our literature review confirms the re-emergence of B/Victoria lineage in Brazil during the years 2000–2002. These data are in line with that of Motta et al.48 which shows the re-emergence of the Victoria-lineage viruses in the Northern hemisphere and in the South and South East regions of Brazil where previously the B/Yamagata lineage was the predominating circulating lineage and the vaccine recommended lineage. Other studies confirm that the B/Yamagata was the major circulating lineage until the 1980s, when B/Victoria lineage viruses appeared; since then, drift variants of both influenza B lineages have been co-circulating worldwide.49,50 As influenza B viruses can co-circulate during an epidemic allowing the re-emergence of old lineages due to re-assortment between the different strains,4,48 there is a need to improve influenza laboratory-based surveillance in Brazil. Importantly these data also highlight the unpredictability of influenza B circulation, making it difficult to consistently predict which B lineage will predominate during a given influenza season.
Due to the little cross-protection conferred between the two antigenically distinct influenza B lineages, a vaccine lineage mismatch with the circulating lineage could result in additional burden in terms of health care resource utilization and can adversely impact the society. This burden could, in particular, be substantial during an outbreak. To avoid this additional burden, a plausible option would be to switch from the use of trivalent influenza vaccines to quadrivalent vaccines in national campaigns.51 Quadrivalent influenza vaccines are expected to offer broader protection against influenza B disease. The WHO recently updated recommendations beginning with the 2013–2014 influenza, now recommending the use of a quadrivalent vaccine composition with the addition of a second influenza B lineage as well as the two influenza A strains and one influenza B lineage contained in the trivalent vaccines.6,52
Some limitations of this review require consideration. Besides the inherent limitations of each of the studies included in the literature search and abstracts, there are other important limitations. Statistical and quality analysis of the articles was not performed. Also, the interpretation of data is constrained by the gaps in laboratory surveillance in which there were no influenza B data available for many years with respect to age, severity, and seasonality. As we present data over many years through which the surveillance system has gradually improved, the number of positive samples is still small, and the more recent years are likely to be over-represented. Additionally, sentinel surveillance data cannot be truly representative for the whole population as the system of sentinel units involves different levels of care. Moreover, there is no standard protocol for patient selection for sample collection which may have led to a selection bias of certain age groups. Despite these limitations, the surveillance system for influenza and other respiratory viruses which has improved over the years has proven useful to describe influenza B circulation patterns for Brazil and demonstrate that influenza B epidemiology is changing in line with observations reported from other countries.4
ConclusionsInfluenza surveillance systems, and laboratory-based and epidemiological studies of influenza B in Brazil, while limited, have improved over the years. There is a need to strengthen and extend influenza surveillance for influenza B sample subtyping to determine the behavior pattern of influenza B lineages. The findings from this integrative data review provide evidence of the unpredictable nature of influenza B circulation in Brazil, the increasing frequency of co-circulation of both influenza B lineages, and mismatch between the circulating influenza B lineage and the composition of the seasonal influenza vaccine for the region. This data is suggestive of the additional benefit that quadrivalent influenza vaccines, containing both influenza B lineages (B/Yamagata and B/Victoria), could offer over the use of the currently available trivalent vaccines for the prevention of seasonal influenza in Brazil.
Authors contributionsAll authors participated to the conception/design of the review, performed or supervised the analysis, and interpreted the data. Otavio Cintra, Eliana Nogueira Castro de Barros, Laís Freitas and Erika Rossetto collected or assembled the data. Erika Rossetto wrote the preliminary report of the review findings. All authors read and approved the final manuscript.
Conflicts of interestEliana Nogueira Castro de Barros, Otavio Cintra and Romulo Colindres are employees of the GSK group of companies. Romulo Colindres and Otavio Cintra report ownership of stock options and/or restricted shares. Laís Freitas reports that she is working for GSK Vaccines, but is employed by Shift de Gestão em Serviços. Erika Rossetto has no conflict of interest.
The authors would like to thank Andreza Madeira Macario (epidemiologist to INOVATEC) for intellectual contribution to the study, Amrita Ostawal for medical writing services (consultant publications writer to GSK Vaccines) and Bruno Dumont (Business & Decision Life Sciences on behalf of GSK Vaccines) for editorial assistance and publication coordination.
On behalf of GSK Vaccines.
GlaxoSmithKline Biologicals SA funded this study and was involved in all stages of study conduct, including analysis of the data and funded all costs associated with the development and publication of this manuscript.