This study was undertaken in order to assess the involvement of Mycobacterium tuberculosis pili (MTP) as an adhesin, invasin, and cytokine inducer in the M. tuberculosis-epithelial cell interaction. A MTP-deficient strain of M. tuberculosis demonstrated a significant reduction of 69.39% (p=0.047) and 56.20% (p=0.033) in its ability to adhere to and invade A549 pulmonary epithelial cells, respectively, in comparison with the wild-type strain. Complementation of the MTP-deficient mutant restored its adhesion and invasion capacity back to the wild-type levels. Overall, it was found that similar concentrations of IL-1β, IL-4, IL-6, IL-8, G-CSF, IFN-γ, MCP-1, and TNF-α were induced in A549 cells infected with the MTP-proficient and MTP-deficient strains. However, at 48h post-infection, the MTP-deficient mutant induced significantly lower levels of TNF-α than the wild-type strain (p=0.033). Furthermore, at 72h post-infection, the mutant induced significantly higher levels of IL-8 than the wild-type (p=0.005). We conclude that MTP is an adhesin/invasin of epithelial cells and, while playing a role in M. tuberculosis entry, they do not appear to largely influence the epithelial cell cytokine response.
Mycobacterium tuberculosis, the notorious causative agent of tuberculosis (TB), remains one of the main causes of human mortality by an infectious disease. The lack of effective treatment, diagnostic, and preventative strategies continue to impede TB control, which is further compounded by the increasing prevalence of multi-, extensively, and totally drug-resistant strains of the pathogen and the HIV/AIDS co-epidemic. A better understanding of the virulence factors that are associated with the pathogenesis of this organism is crucial to improve strategies to control and reduce global TB burdens.
Adhesins are molecules or structures that are present extracellularly on the bacterial cell surface. They play an important role in the initial host-pathogen interaction by mediating adherence, a precursor to host cell entry. A major M. tuberculosis adhesin of epithelial cells is the heparin-binding hemagglutinin adhesin (HBHA), which has also been implicated in extrapulmonary dissemination of the pathogen.1 Bacterial species are known to produce multiple adhesins to effectively infect host cells. Therefore, other adhesins may contribute to M. tuberculosis adhesion to epithelial cells.2
Pili are a well-characterized family of bacterial adhesins. Two pili types have been described for M. tuberculosis, namely curli and type IV pili.3 The curli or coiled pilus morphotype, first identified in Escherichia coli and Salmonella spp., are known to be mediators in biofilm formation, adherence to and colonization of the host, and induction of host inflammation.4 The curli-like pili of M. tuberculosis, MTP, identified by Alteri et al.,5 resemble the pili fibers of E. coli and Salmonella enterica and are encoded by the mtp (Rv3312A) gene. These researchers also reported on the ability of MTP to bind to the extracellular matrix protein laminin in vitro, which suggested that MTP may function as an adherence factor.5
A central focus of our research group currently is elucidating the function of MTP. We have previously identified the role of MTP in in vitro biofilm formation6 and in the adherence to and invasion of macrophages.7 We have also shown that the mtp gene sequence is unique to M. tuberculosis complex strains and highly conserved amongst clinical isolates and, thus, MTP may be a suitable biomarker for a TB diagnostic test.8
In this study, we compared adhesion and invasion of a wild-type, Δmtp mutant, and mtp-complemented M. tuberculosis strains to the A549 pulmonary epithelial cell line. We also compared cytokine production by A549 cells infected with these strains.
Materials and methodsBacterial strains and growth conditionsM. tuberculosis clinical isolate V91249 and the previously constructed MTP-deficient Δmtp mutant and MTP-overexpressing mtp-complemented strains6 were used in this study. Cultures were grown at 37°C in Middlebrook 7H9 broth (Difco), supplemented with 10% (v/v) oleic acid-albumin-dextrose-catalase (OADC; Becton Dickinson), 0.5% (v/v) glycerol (Sigma), and 0.05% (v/v) Tween 80 (Sigma). Hygromycin (75μgmL−1; Roche Applied Sciences) or kanamycin (30μgmL−1; Sigma) were included for the mutant and complemented strain cultures, respectively.
Epithelial cell preparationThe A549 human type II alveolar epithelial cell line (ATCC CCL-185) was maintained in Eagle's Minimum Essential Medium with Earle's Balanced Salt Solution and 2mM l-glutamine (Lonza), supplemented with 10% (v/v) fetal bovine serum (Biowest). Following trypsinization, 2×105 cells were seeded into 24-well plates (NEST Biotechnology) and incubated for 24h at 37°C in 5% CO2.
Infection of epithelial cellsLogarithmic phase mycobacterial cultures were pelleted by centrifugation (2000×g for 10min; Heraeus Multifuge 3S-R Centrifuge, Thermo Scientific) and resuspended in fresh cell culture media. The epithelial cells were infected with the bacterial strains at a multiplicity of infection (MOI) of 1:1 for the adhesion and invasion assays. A MOI of 10:1 was used for the cytokine assay to ensure sufficient cytokine production.10 Plates were incubated at 37°C and 5% CO2 at the desired time intervals. The inocula were also serially diluted and plated in triplicate onto Middlebrook 7H11 (Difco) agar plates, containing 10% (v/v) OADC and 0.5% (v/v) glycerol, to enumerate viable bacilli used for infection. Trypan blue exclusion was used to determine the number of viable epithelial cells at the time of infection, to confirm the MOI.
Adhesion assayThe adhesion assay was performed as previously described.7 At the end of the 1h incubation period, media was removed and the epithelial cells washed thrice with 1mL phosphate-buffered saline (PBS; Oxoid). Adherent epithelial cells were lysed with 1mL of 0.1% (v/v) Triton X-100 (Sigma) for 20min at 37°C and 5% CO2. The number of adherent bacteria was determined by plating ten-fold serial dilutions of the lysate, in triplicate, onto 7H11 agar plates. Colonies were counted after 3 weeks of incubation at 37°C.
Invasion assayThe invasion assay was performed as previously described.7 After 2h incubation, monolayers were treated with amikacin (300μgmL−1; Sigma) for 1h at 37°C in a 5% CO2 atmosphere. The media was removed and plated onto 7H11 plates to confirm the death of non-invaded microbes. The amikacin was removed by washing thrice with PBS. Adherent epithelial cells were lysed and the number of viable invaded organisms was determined as in the adhesion assay.
Cytokine analysisFollowing 4h of infection, spent media was removed, the wells washed with PBS, and fresh tissue culture media added. At 24, 48, and 72h post-infection, cell culture supernatants were removed and filtered through a 0.2μm filter (PALL Life Sciences). Bovine serum albumin (BSA; Sigma) was added at a final concentration of 0.5% (w/v) and the samples were stored at −80°C. The cytokine levels in supernatants were measured using a Bio-Plex Pro Human Cytokine Multi-Plex Panel (Bio-Rad) in a Bio-Plex 200 System (Bio-Rad), according to the manufacturer's instructions.
Statistical analysisAdhesion/invasion assays were performed at least three independent times in duplicate. Cytokine experiments were performed at two independent times and assayed in duplicate. The significance of differences between the strains was determined by one-way analysis of variance (ANOVA). All data were analyzed using SPSS 22.0 statistical software. p<0.05 was considered significant.
Results and discussionThe entry of tubercle bacilli into the alveolar space of the lungs is followed by the invasion or internalization of the organism by alveolar macrophages.11 This interaction has important implications for the pathogen's survival and replication, evasion of the host immune response, and in its dissemination. Although less widely studied, M. tuberculosis also has the ability to invade and replicate in epithelial cells.12–14 Mehta et al.13 showed that whilst M. tuberculosis invades macrophages more efficiently than epithelial cells, the intracellular replication within the latter cell type was significantly higher. Epithelial cells may, therefore, provide a less harsh intracellular environment for the pathogen than macrophages. Furthermore, they are present in greater numbers than macrophages within alveoli15 and, thus, may represent the first host cell type that the bacilli encounter in the lung.
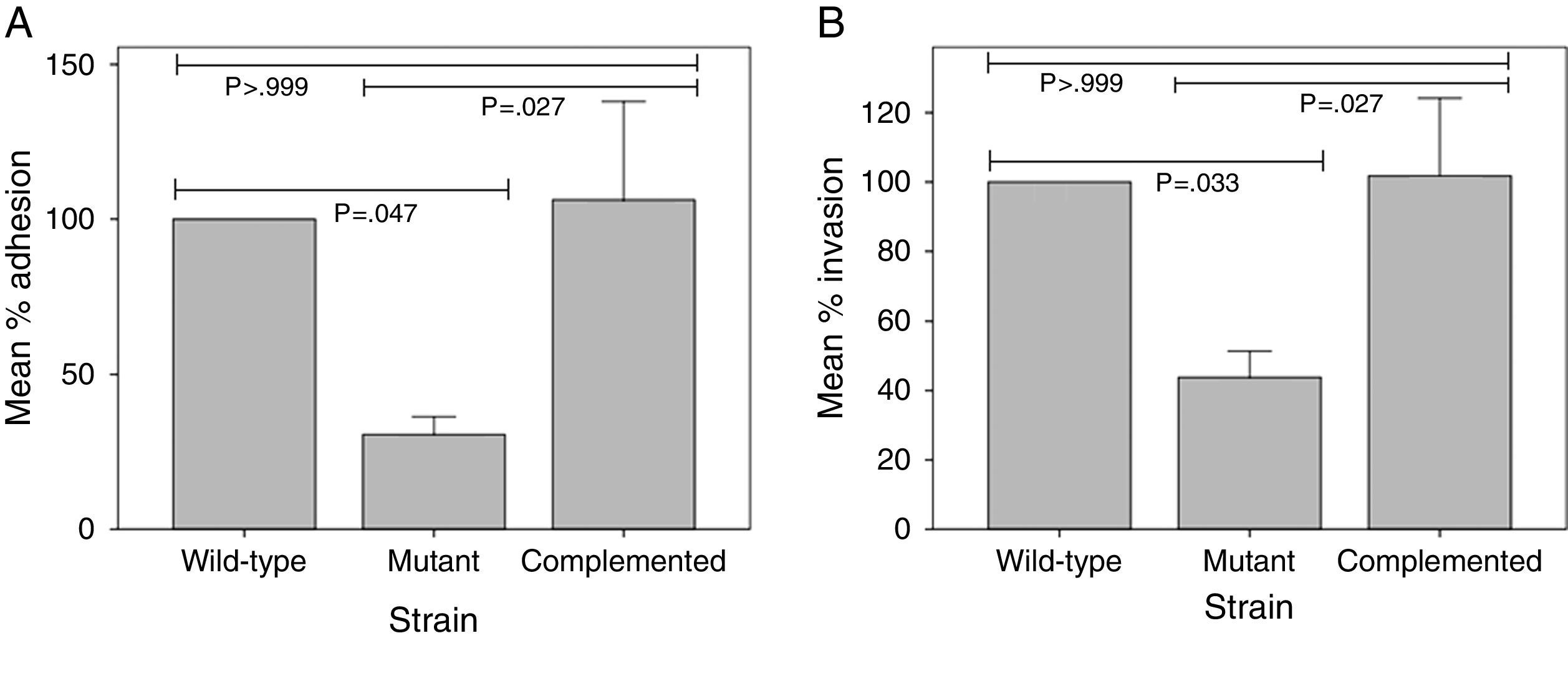
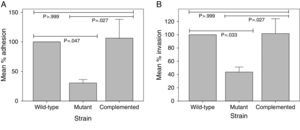
Using immunofluorescence microscopy with anti-MTP antibodies, Alteri16 detected expression of MTP in a previous study with A549 epithelial cells. This finding suggested a possible role of MTP in the M. tuberculosis-epithelial cell interaction. To ascertain whether MTP enable M. tuberculosis entry into epithelial cells, we compared adhesion and invasion capabilities of a MTP-deficient and a MTP-overexpressing M. tuberculosis strain in A549 cells. MTP-deficiency significantly diminished adhesion to and invasion of A549 cells compared with the parental strain, by 69.39% (p=0.047) and 56.20% (p=0.033), respectively (Fig. 1). Complementation restored adhesion (p=0.027) and invasion (p=0.027) to the wild-type levels (Fig. 1). Increased MTP expression by the complemented strain did not, however, significantly alter adhesion (p>0.999) to and invasion (p>0.999) of epithelial cells compared with the wild-type (Fig. 1).
The role of M. tuberculosis pili (MTP) in the adhesion (A) to and invasion (B) of epithelial cells. A549 epithelial cells were infected with a wild-type, MTP-deficient mutant, and MTP-overexpressing complemented strains for 1h (adhesion assay) or 2h (invasion assay). Amikacin-treated and -untreated infected epithelial cells were washed, lysed, and plated onto agar plates to enumerate viable invaded and adherent bacilli, respectively. Results are expressed as the mean percentage adhesion to or invasion of the mutant and complemented strains, relative to that of the wild-type. Error bars are ±1×SEM.
Our findings are similar to those of other studies, which have shown that curliated bacteria are better able to adhere to and enter epithelial cells than non-curliated bacteria. Curliated E. coli strains were reported to be better at adhering to uroepithelial cells17 and human laryngeal epithelial (Hep-2) cells.18 They also displayed increased invasion of human cervical epithelial (HeLa) cells19 and Hep-2 cells.20 Similarly, curliated Salmonella typhimurium promoted attachment to cultured mouse small intestinal epithelial cells.21
The current results differ from our previous study, which found that the adherence of M. tuberculosis to THP-1 macrophages was dependent on the density of piliation.7 The complemented strain expressed a 51-fold higher mtp expression than the wild-type, driven by the constitutively expressed hsp60 promoter of the pMV261 vector used for complementation.6 This MTP-overexpression significantly increased adhesion to THP-1 macrophages, but not A549 epithelial cells, compared with the wild-type. This observation may possibly indicate different mechanisms or host receptor interaction of MTP with these two host cell types or, alternatively, varying expression of the same host cell receptor between macrophages and epithelial cells.
The only definitive adhesin/invasin previously described for M. tuberculosis is the HBHA. Pethe et al.1 reported a 60% and 80% reduction in the adhesion to and invasion of A549 pneumocytes, respectively, by a HBHA-deficient strain compared with the wild-type. Loss of HBHA did not influence adhesion to and invasion of macrophages.1 Due to the significant reduction in adherence to and invasion of epithelial cells, HBHA was regarded as the principal adhesin of epithelial cells. However, our study confirms that M. tuberculosis possesses more than a single adhesin, with a redundant function, which may account for the success of the pathogen. Therapeutic strategies targeting multiple adhesins may, therefore, prove most beneficial to incapacitating the pathogen.
In addition to serving as an adhesin and invasin of epithelial cells, we previously reported a 42% and 69% decrease in adhesion to and invasion of THP-1 macrophages, respectively, by the MTP-deficient strain.7 The difference in adhesion and invasion rates suggest that MTP may play a more significant role as an adhesin in epithelial cells than in macrophages, whilst the opposite may be true for its function as an invasin.
Pethe et al.1 suggested that following entry and replication within macrophages, M. tuberculosis enters non-phagocytic cells, which facilitate extrapulmonary dissemination. The function of MTP in both phagocytic and non-phagocytic host cell interactions implies that MTP is a major adhesin and participates in multiple stages of M. tuberculosis pathogenesis. During host colonization, bacteria are also known to develop into structured microbial communities or biofilms, which are associated with drug tolerance.22 In addition to serving as an adhesin of host cells, MTP mediate the formation of in vitro M. tuberculosis biofilms.6
Given the collective data on both adhesins, it seems that MTP may be a marginally better adhesin than HBHA and that the latter may, in turn, be more effective as an invasin compared with the former. This can only be confirmed by testing these deletion mutants under the same experimental conditions. However, both these adhesins/invasins appear to play major roles in the virulence of this pathogen and suggest that in combination, they may represent powerful targets for adjunctive immuno- or chemotherapy or potential vaccine development.
The outcome of M. tuberculosis infection is dependent on both the virulence of the pathogen and the host cellular defence mechanisms. M. tuberculosis-infected epithelial cells are known to secrete cytokines and chemokines, including interleukin-8 (IL-8) and monocyte chemotactic protein-1 (MCP-1), which contribute to the host inflammatory response.10 Pili do not only function in the host-pathogen interaction by enabling adhesion to and invasion of host cells, but the interaction of pili with host cell receptors is essential to the induction of inflammation via the production of inflammatory markers, such as cytokines.23 Several studies have demonstrated that pathogen-induced inflammation on epithelial cells can be attributed to pathogen-associated molecular patterns, such as pili or flagella, which stimulate pro-inflammatory signals.24–27 In order to determine whether MTP are activators of the epithelial cell cytokine response, we compared the in vitro cytokine profiles of A549 cells infected with the wild-type, Δmtp mutant, and complemented strains, using a multiplex assay.
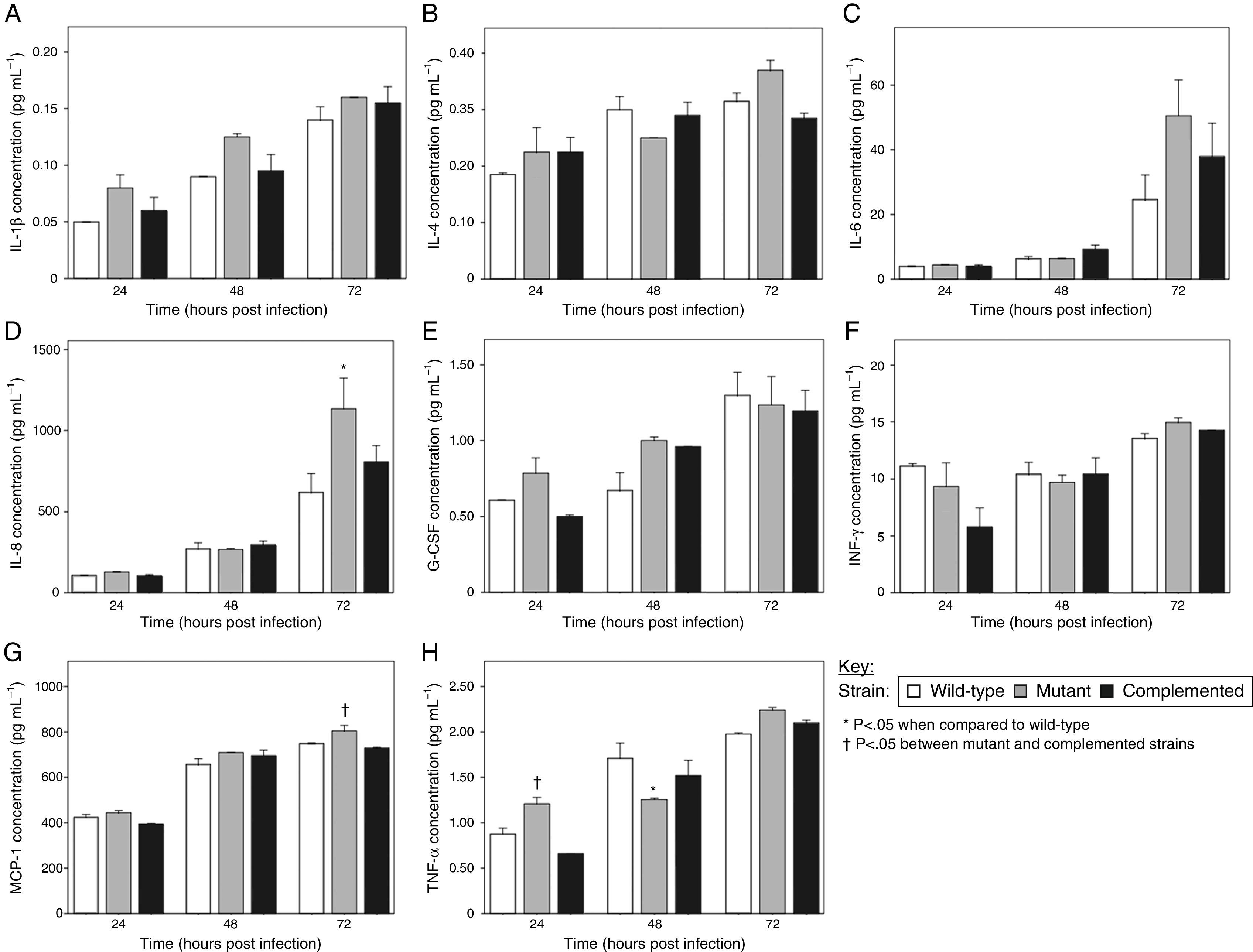
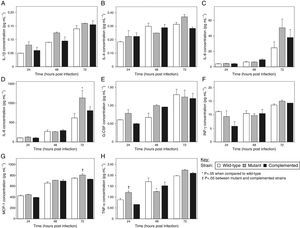
The A549 cells were found to produce IL-1β, IL-4, IL-6, IL-8, granulocyte colony-stimulating factor (G-CSF), interferon-gamma (IFN-γ), MCP-1, and TNF-α in this study, although at low concentrations for some analytes. Titers for IL-1β, IL-4, IL-6, G-CSF, and IFN-γ were not significantly different for the epithelial cells infected with the wild-type, mutant, and complemented strains at all three time points tested (Fig. 2a, b, c, e and f). Similarly, there were no significant differences in IL-8, MCP-1, and TNF-α levels elicited by the MTP-deficient and MTP-proficient strains at most of the time intervals, with a few exceptions (Fig. 2d, g and h). The only indication that MTP induce the cytokine response was the observation that at 48h post-infection, the mutant strain displayed a significant reduction in TNF-α production compared with the wild-type (p=0.033) (Fig. 2h). However, at 24 and 72h post-infection, the mutant strain showed, on average, higher levels of TNF-α induction than both the wild-type and complemented strains (Fig. 2h). The mutant strain also induced the lowest levels of IL-4, IL-8, and IFN-γ at 48h post-infection compared with the MTP-proficient strains, but this did not reach statistical significance (Fig. 2b, d and f).
The role of M. tuberculosis pili (MTP) in epithelial cell cytokine production. A549 epithelial cells were exposed to the wild-type, MTP-deficient mutant, and MTP-overexpressing complemented strains for 4h. Extracellular bacteria were removed by washing, fresh media added, and cell culture supernatants at 24, 48, and 72h post-infection were assayed for levels of the cytokines IL-1β (A), IL-4 (B), IL-6 (C), IL-8 (D), G-CSF (E), IFN-γ (F), MCP-1 (G), and TNF-α (H), using a Bio-Plex assay (Bio-Rad). Error bars are ±1×SEM.
These findings suggest that, apart from TNF-α (and to a lesser extent IL-4, IL-8, and IFN-γ) at 48h post-infection, MTP-mediated entry into epithelial cells does not trigger responses of the cytokines tested. These findings contrast other studies that have associated curli pili as inducers of the immune system. Tükel et al.28 showed that S. enterica serotype Typhimurium curli are recognized by Toll-like receptor 2, resulting in the activation of IL-8. Bian et al.29 demonstrated that curliated E. coli result in the activation of IL-6, IL-8, and TNF-α. Macrophages and human embryonic kidney cells, and not epithelial cells, were the host cell models in these studies. However, others have demonstrated an association between bacterial invasion and cytokine production by epithelial cells.30–32 Interestingly, the mutant strain induced significantly higher levels of IL-8 than the wild-type at 72h post-infection (p=0.005) (Fig. 2d). In addition, significantly more MCP-1 (p=0.048) and TNF-α (p=0.004) were secreted by mutant-infected epithelial cells at 72 and 24h post-infection, respectively, compared with the complemented strain (Fig. 2g and h). Whilst not reaching statistical significance, the mutant strain showed, on average, elevated levels of all cytokines detected in this study, except IL-4 and IFN-γ, at 24h post-infection than the wild-type and complemented strains (Fig. 2). Similarly, at 72h post-infection, only the G-CSF concentrations were not the highest for the mutant strain samples (Fig. 2). In addition, at 48h post-infection, the mutant strain induced the highest concentrations of IL-1β, G-CSF, and MCP-1 (Fig. 2a, e and g). It is, therefore, tempting to speculate that MTP-mediated invasion of epithelial cells may confer an advantage to the pathogen by dampening the epithelial cell cytokine response, which is a key factor in infection control. This would represent an additional strategy of M. tuberculosis to ensure survival by escaping the host immune system. Alternately, the decreased cytokine levels may be attributed to increased cytotoxicity of wild-type and complemented strain-infected epithelial cells, due to the higher bacterial loads than for the mutant-infected epithelial cells. It has recently been reported that a positive correlation exists between M. tuberculosis adhesion to and invasion of epithelial cells and its cytotoxic effect.33 Another possible reason for this phenomenon could be that MTP-deficiency triggers the expression of other adhesins, which induce cytokine production. Future cytotoxicity studies and cytokine assays on epithelial cells treated with purified MTP protein would help to verify these postulates.
To conclude, we have shown that MTP function in the adherence and entry of M. tuberculosis into epithelial cells. However, MTP did not largely influence the overall cytokine responses of M. tuberculosis-infected epithelial cells. Future in vivo studies will address the importance of these in vitro findings.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the National Research Foundation of South Africa and the Canon Collins Educational and Legal Assistance Trust for financial support. We also thank Dr. Lenine Liebenberg (CAPRISA) for assistance with the cytokine data analysis.