Pegylated interferon alpha (Peg IFN-α) in combination with ribavirin is the backbone of treatment in chronic hepatitis C (CHC). Cardiotoxicity due to interferon therapy is rare. The most frequent cardiovascular complications are arrhythmias and ischemic manifestations. Cardiomyopathy is extremely rare but can be life threatening. We present the case of a 41-year-old female patient with CHC in whom Peg IFN-α induced dilated cardiomyopathy and hypothyroidism. Chest radiography showed an enlarged and globular cardiac silhouette and pulmonary congestion. Echocardiography showed decreased left ventricular systolic function with an ejection fraction of 32% and fractional shortening of 15%. Cardiomyopathy had a complete remission after cessation of antiviral therapy with short-term heart failure medications and supportive care. Then we review the current literature about interferon induced cardiomyopathy in patients with HCV infection, as well as share our clinical experience in diagnosing and managing this rare complication.
Hepatitis C virus (HCV) is a major public health problem, involving approximately 170 million people worldwide.1 Pegylated interferon-α (Peg IFN-α) in combination with ribavirin is the backbone of treatment in patients with HCV infection before direct-acting antiviral agents (DAAs) became available, and still is the optimal choice for the majority of patients with CHC. Interferon-α (IFN-α)-induced thyroid dysfunction (TD) has been well documented, with an incidence ranging from 10% to 27.7%.2–6 Cardiovascular complications caused by interferon consists mainly of arrhythmia, myocarditis, reversible hypertension, ischemic heart disease, pericarditis and pericardial effusion.7–10 However, few cases of interferon-induced cardiomyopathy have been reported.7,8,11–13 We herein report a patient with CHC in whom previous standard IFN-α plus ribavirin had not induced severe complications, while Peg IFN-α did induce dilated cardiomyopathy and hypothyroidism. Cardiomyopathy had a complete remission after cessation of Peg IFN-α and ribavirin. However, permanent thyroid hormone replacement therapy is needed to treat hypothyroidism.
Case presentationA 41-year-old woman without history of heart disease was admitted to our hospital with 2-week history of gradually increasing dyspnea and fatigue in June 2008. She had a history of rheumatoid arthritis for 10 years and was not receiving any medications. Chronic hepatitis C had been diagnosed in 2006, and HCV genotype was 2a. After initial therapy with IFN-α-2b (3MU, 3 times weekly) and ribavirin (800mg/d) for six months, HCV RNA was undetectable by polymerase chain reaction at the end of treatment and six months follow-up. In January 2008, the patient consulted for CHC when she began to experience generalized malaise and fatigue. The patient's baseline chest radiograph showed normal lung fields and no cardiomegaly, and an electrocardiogram was normal. Laboratory tests revealed an alanine aminotransferase (ALT) level of 174U/L (normal up to 40U/L), aspartate aminotransferase (AST) level of 123U/L (normal up to 40U/L), and positive serology for HCV. The HCV RNA level was 546,000IU/mL. The patient refused liver biopsy prior to the second course of interferon therapy. Combination therapy was initiated with Peg IFN-α-2a at the dose of 180μg per week in combination with ribavirin at the dose of 800mg daily. The patient responded well, her aminotransferase levels normalized, and HCV RNA was undetectable at week 4. After the twelfth week of treatment, the patient was treated with levothyroxine for hypothyroidism without discontinuation of antiviral therapy.
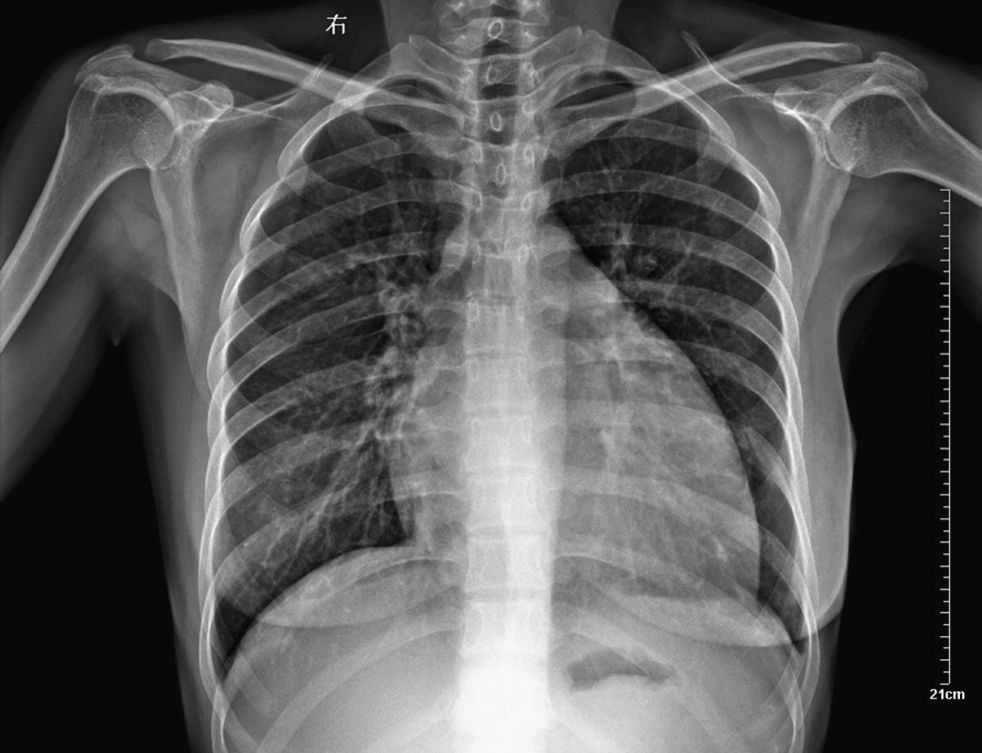
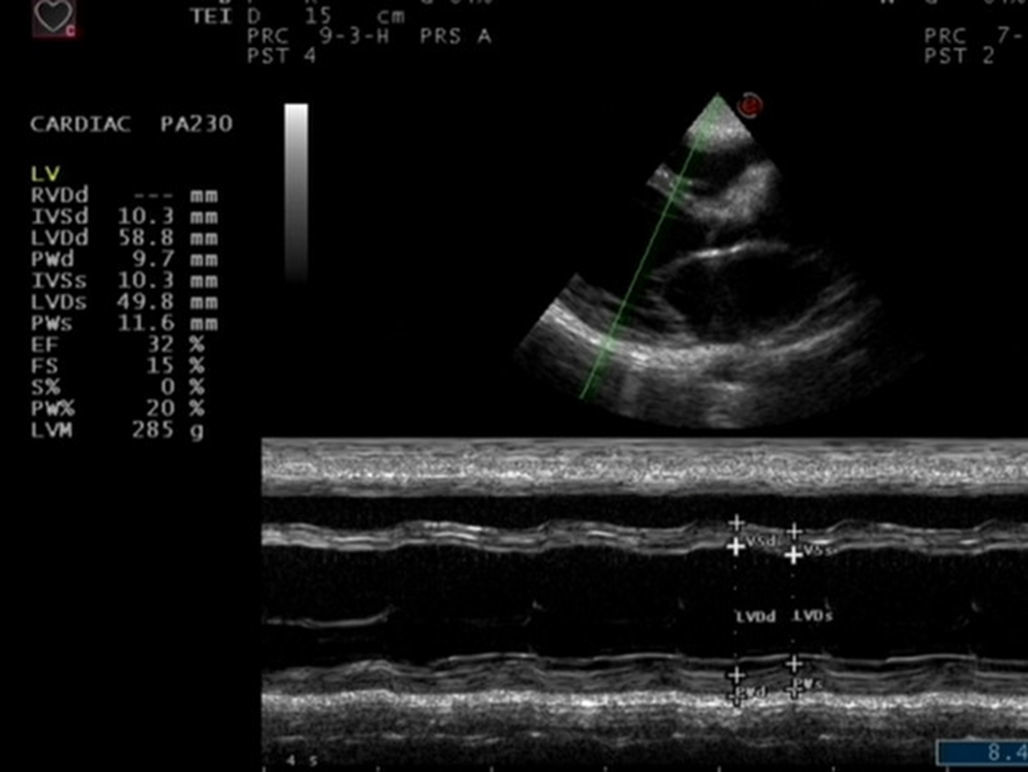
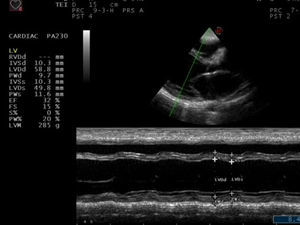
Physical examination revealed blood pressure of 130/90mmHg, heart rate of 122/min, a dilated jugular vein, and an audible gallop rhythm and systolic ejection murmur. There was mild peripheral edema. Chest radiography showed an enlarged and globular cardiac silhouette and pulmonary congestion without pleural effusion (Fig. 1). Serum free T3, T4, total T3, T4 and thyroid stimulating hormone (TSH) were within normal values with levothyroxine replacement therapy at a dose of 50μg daily. Cardiac enzymes, liver enzymes, erythrocyte sedimentation rate and C reactive protein were normal. Antinuclear, antithyroperoxidase and antithyroglobulin antibodies were positive. Rheumatoid factor and anti-α-myosin antibodies were absent. Coxsackie virus, Chlamydia pneumoniae and Mycoplasma pneumoniae serology were negative. Echocardiography showed decreased left ventricular systolic function with an ejection fraction (EF) of 32% and fractional shortening (FS) of 15%, as well as dilated left ventricle with decreased wall motion of entire left ventricle (Fig. 2). Dilated cardiomyopathy and hypothyroidism secondary to Peg IFNα-2a was suspected. Therefore, Peg IFN-α and ribavirin was discontinued after 23 weeks of treatment. The patient was treated with Benazepril, Digoxin, Furosemide, Metoprolol Succinate and Levothyroxine. Her symptoms improved in one week and she was discharged on the same medications. A second echocardiogram three months later showed an EF of 47% with normal chambers, and heart failure medications were discontinued. She maintained normal aminotransferases and undetectable HCV RNA after 24 months of follow up. However, she needs permanent thyroid hormone replacement therapy for hypothyroidism.
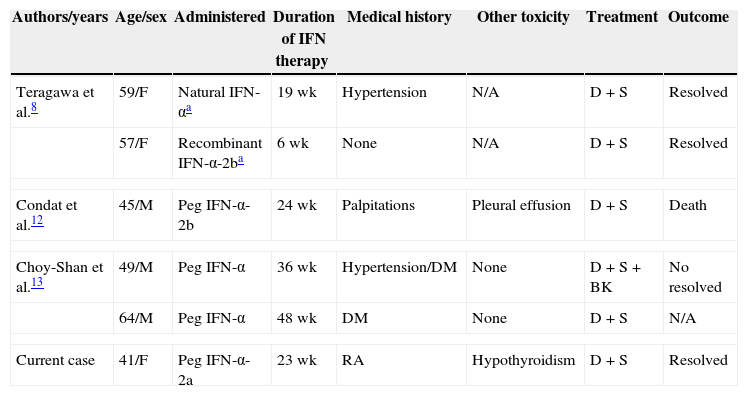
Peg IFN-α in combination with ribavirin was the historical backbone of treatment in patients with HCV infection in the past decade, and will still be the optimal choice for the majority of patients with CHC. As a result, the safety of combination therapy is going to remain a major issue for patients with CHC. Although cardiovascular complications from IFN-α are scarce, the growing number of patients receiving Peg IFN-α therapy will undoubtedly lead to an increasing number of patients with cardiotoxicity. Out of these complications, arrhythmia and ischemic heart disease are the predominant types, followed by myocarditis and reversible hypertension, while cardiomyopathy is rare.7,8 To the best of our knowledge, only six cases of cardiomyopathy associated with IFN-α treatment for chronic hepatitis C has been described to date in English literature, including our case.7,12,13 The clinical data of the six cases are shown in Table 1.
Case reports of cardiomyopathy associated with interferon-α therapy for HCV in the literature.
| Authors/years | Age/sex | Administered | Duration of IFN therapy | Medical history | Other toxicity | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Teragawa et al.8 | 59/F | Natural IFN-αa | 19 wk | Hypertension | N/A | D+S | Resolved |
| 57/F | Recombinant IFN-α-2ba | 6 wk | None | N/A | D+S | Resolved | |
| Condat et al.12 | 45/M | Peg IFN-α-2b | 24 wk | Palpitations | Pleural effusion | D+S | Death |
| Choy-Shan et al.13 | 49/M | Peg IFN-α | 36 wk | Hypertension/DM | None | D+S+BK | No resolved |
| 64/M | Peg IFN-α | 48 wk | DM | None | D+S | N/A | |
| Current case | 41/F | Peg IFN-α-2a | 23 wk | RA | Hypothyroidism | D+S | Resolved |
Peg IFN-α, pegylated interferon alpha; DM, diabetes mellitus; RA, rheumatoid arthritis; D, discontinuation pegylated interferon alpha; S, supportive care; BK, biventricular pacemaker; N/A, data not available.
The present patient was the first case to develop cardiomyopathy and hypothyroidism after standard of care antiviral treatment for HCV infection. In this case, Peg IFN-α may have been responsible for the onset of cardiomyopathy. Arguments in support of a correlation between Peg IFN-α and cardiomyopathy include the following aspects: all other possible infectious and drugs or systemic causes were ruled out; cardiotoxicity was present during Peg IFN-α treatment; cardiotoxicity regression after drug withdrawal; absence of any previous heart disease history and specific investigations before treatment excluding a pre-existing cardiomyopathy, and the symptoms and the results of the various investigations carried out in the context of the dilated cardiomyopathy. We used the causal criteria from the World Health Organization to show the probability of adverse drug reaction.14
The mechanism behind IFN-α induced cardiotoxicity was not yet clarified. In reported cases, the dose of standard IFN-α was always higher than three million units, three times a week, and in the majority of cases very high doses were used.7,8,10,11 Cardiomyopathy associated with standard IFN-α treatment for CHC is extremely rare, maybe due to the lower doses. The only two reported cases were administration of IFN-α at a dose of six million units daily for the first two weeks and six million units three times weekly thereafter.8 Our patient present cardiomyopathy during Peg IFN-α rather than standard IFN-α therapy. Whatever the mechanism, it is conceivable that pegylated interferon provides higher levels of interferon plasma concentration in a continuous fashion because of prolonged half-life, and thus could bear a higher risk of cardiomyopathy. On the other hand, there are no described predisposing factors for interferon cardiotoxicity. Studies have shown that combination therapy with Peg IFN-α and ribavirin does not cause a significant deterioration in cardiac function in patients without cardiac disease15 and may be safely offered to carefully selected chronic hepatitis C patients with coexisting, clinically significant heart disease,16 indicating that the diverse genetic predisposition of the individuals may substantially contribute to cardiotoxicity.
Cardiomyopathy induced by standard IFN-α was always reversible when there was no concomitant factor.7,8,11 Conversely, Peg IFN-α related cardiomyopathy showed a poor prognosis.12,13 Although our case had complete resolution of the cardiomyopathy, one case died due to cardiogenic shock12 and another case had irreversible ventricular dysfunction and remains symptomatic despite maximal medical therapy and implant of a biventricular pacemaker.13
TD is a relatively common adverse reaction to IFN-α based therapy and it also represents an extra-hepatic manifestation of HCV infection.3,5 Studies have shown that the incidence of TD associated with IFN-α therapy differs markedly between racial and ethnic groups and fibrosis stages, with an incidence ranging from 10% to 27.7%.2–6 The majority of TD is hypothyroidism, and more than half of them return spontaneously to normal after cessation of antiviral therapy.3–6 However, a few patients, as well as our case, need permanent thyroid hormone replacement therapy.3–6 Fortunately, it does not always necessitate alteration or discontinuation of therapy due to TD,2–6,17 and it also does not impact the response to combination therapy with interferon and ribavirin.3,17
ConclusionWe report a patient without any history of cardiac disease who developed dilated cardiomyopathy and hypothyroidism after Peg IFN-α plus ribavirin treatment. Cardiomyopathy is an exceptional but unanticipated complication of antiviral therapy for HCV infection. It may be associated with the diverse genetic predisposition of individuals. Prompt discontinuation of HCV medication is mandatory, and short-term heart failure medications together with supportive care are essential. Clinicians should be aware of this potentially cardiovascular complication and the possible acuity of its onset. We suggest that periodic monitoring of the cardiac function is necessary, especially for symptomatic patients, to detect early toxic effects during interferon administration.
Financial supportThis work was supported by Personnel training special funds of the Second Affiliated Hospital, College of Medicine, Xi’an Jiaotong University [RC(XM)201105] and The Health Research Foundation of Shaanxi Province (No. 2010H31).
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by Personnel training special funds of the Second Affiliated Hospital, College of Medicine, Xi’an Jiaotong University [RC(XM)201105] and The Health Research Foundation of Shaanxi Province (No. 2010H31).