Assessing health-related quality of life is an important aspect of clinical practice. Thus, the present study attempts to assess the health-related quality of life of patients with chronic liver disease.
MethodsA cross-sectional survey was conducted on 133 chronic liver disease patients, using three instruments: a demographic questionnaire, the Chronic Liver Disease Questionnaire, and Model for End-Stage Liver Disease index. Variables were expressed as frequencies, percentages, means, and standard deviations. The statistical analysis included Pearson's correlation, Student's t-test, and analysis of variance (p<0.05 was considered significant).
ResultsThe mean age of included subjects was 50.5±13.3 years. The majority were male (66.2%), Caucasian (70.7%), and had a family income of US$329–US$658.2. Over half of the patients (56.4%) were infected by hepatitis C virus and 93.2% had low Model for End-Stage Liver Disease scores. Model for End-Stage Liver Disease score was related to age (r=0.185; p=0.033). Higher mean Chronic Liver Disease Questionnaire scores were obtained for emotional function (39.70/SD±12.98) and while lower scores were obtained for abdominal symptoms (16.00/SD±6.25). Fifty-two patients (39.1%) presented overall low (<5) Chronic Liver Disease Questionnaire scores. Furthermore, Chronic Liver Disease Questionnaire score was related to family income (r=0.187, p=0.031).
ConclusionMost individuals presented high mean Chronic Liver Disease Questionnaire scores, indicating low health-related quality of life, especially individuals with low family income.
Chronic diseases in general are increasingly widespread,1 and diseases of the liver in particular are considered a global public health problem.2 Chronic liver disease (CLD) substantially contributes to mortality and morbidity rates.3,4 Worldwide, about 500 million individuals have CLD with a viral etiology.5 However, CLD also has non-viral etiology including alcoholic hepatitis, fatty liver, autoimmune hepatitis, cryptogenic hepatitis, and other unidentified causes.
Over the last few decades, assessing quality of life (QoL) of individuals with diseases has become common clinical practice,6,7 as a consequence of increased survival of patients with chronic diseases.8 The term health-related quality of life (HRQoL) reflects the impact of the disease upon a person's quality of life. It is a subjective, multidimensional concept addressing various aspects of the individuals’ life such as age, gender, socioeconomic status, and type of illness, and treatment,9 that should be considered during patient evaluation.10
CLD has a negative impact on HRQoL since patients often present asthenia, indisposition, abdominal, muscle, and/or joint pain or discomfort, lack of appetite, insomnia, and complications related to liver cirrhosis, such as ascites, variceal bleeding in the stomach and esophagus, and hepatic encephalopathy. Moreover, CLD is linked to job loss, impaired functioning, mood swings, anxiety, low self-esteem, depression, and other emotional problems that severely affect QoL and well-being.11–15
Globally, studies on HRQoL of patients with CLD have used generic and specific instruments.16,17 Recently, a Japanese version of the Chronic Liver Disease Questionnaire (CLDQ) to assess HRQoL was validated in patients with chronic viral hepatitis.18 To the best of our knowledge, this is the first study that assesses the HRQoL of Brazilian patients with CLD using a specific instrument that has been recently translated and validated for this population.19
MethodsPatientsPatients with CLD referred to the Outpatient Clinic of CLD at Hospital das Clinicas of the Federal University of Uberlândia, Minas Gerais State, Brazil in the period of 2011–2012 were recruited for the study. Patients of either gender had be 18 years or more and have a diagnosis of CLD by a hepatologist.
The diagnosis of chronic hepatitis B virus (HBV) infection was based on the presence of hepatitis B surface antigen for more than six months and elevated serum alanine aminotransferase levels with or without HBV DNA, detected by real-time polymerase chain reactions (PCR).20
Chronic hepatitis C virus (HCV) infection was diagnosed based on the presence of hepatitis C antibodies for more than six months assessed by ELISA III and HCV RNA by qualitative polymerase chain reaction and elevated serum alanine aminotransferase levels.21
Primary biliary cirrhosis was diagnosed based on positive anti-mitochondrial antibodies (AMA) and elevated liver enzymes with or without liver biopsy.22 Alcohol was deemed the cause of chronic liver disease if daily alcohol consumption was greater than 40g for at least 10 years with elevated γ-glutamyl transferase when controlling for other liver diseases.23
Autoimmune hepatitis diagnosis was based on simplified diagnostic criteria for routine clinical practice including age, sex, autoantibodies [smooth muscle actin (SMA), anti-nuclear antibody (ANA), AMA, liver-kidney microsomal antibodies (LKM), soluble liver/liver-pancreas antibodies (SLA/LP)], γ-globulins, immunoglobulin A, immunoglobulin G (IgG), immunoglobulin M (IgM), absence of viral hepatitis, and liver histology.24
Diagnosis of nonalcoholic fatty liver disease (NAFLD) was based in the evidence of hepatic steatosis, either by imaging or histology, controlling for other causes of secondary hepatic fat accumulation such as significant alcohol consumption, steatogenic medication, or hereditary disorders.25 Cryptogenic chronic hepatitis was diagnosed if the patient presented with persistent inflammation of the liver, unexplained by conventional clinical, laboratory, and histological methods.26
Liver cirrhosis diagnosis was based on clinical, biochemical, serologic, and radiographic parameters and ultrasonography.27 Cirrhosis without a diagnosis of the etiology was termed “other forms of cirrhosis or unspecified cirrhosis”.
Exclusion criteria were presence of malignancy, liver transplantation, co-infection with human immunodeficiency virus (HIV), psychiatric or emotional problems, cognitive or language difficulties that prevented the reliable application of the questionnaire, and a diagnosis of hepatic encephalopathy.6 The study was conducted in accordance with the Helsinki Declaration and study protocols were approved by local ethics committees. All patients provided informed consent before enrollment.
A pilot study to verify the applicability of these instruments was first conducted on 23 individuals with the same population characteristics. These patients were not included in the final sample.
Data collectionThe investigators informed patients about the study aims. After obtaining informed consent, participants completed a socio-demographic questionnaire and the CLDQ.
The CLDQ has recently been translated and validated for the Brazilian population.18 This instrument was developed by Yonossi et al. in 1999 to assess QoL of patients with liver diseases.6 The CLDQ is the only validated instrument for the different etiologies and degrees of severity of liver disease. It yields both domains and total scores, demonstrating both multidimensional and overall perception of QoL, emphasizing the effects of liver disease symptoms. The Brazilian version comprises 29 questions encompassing six domains: Abdominal Symptoms (AS), Fatigue (FA), Systemic Symptoms (SS), Activity (AT), Emotional Function (EM), and Worry (WR). AS and AT domains have 3 items each; FA, SS, and WR have 5 items each; whereas EM comprises 8 items. Each item is rated on a 7-point Likert scale.28 Higher score on the questionnaire is indicative of minimum symptoms and lower score indicates more pronounced symptoms. A CLDQ cut-off score was determined to evaluate HRQoL based on prior literature; mean CLDQ scores ≥5 was considered to represent high HRQoL and mean CLDQ scores <5 implied low HRQoL.29
The Model for End-Stage Liver Disease (MELD) index—a mathematical model that uses three laboratory tests (values of serum creatinine, serum total bilirubin, and international normalized ratio; INR) to determine the severity of liver disease30—was also calculated. The formula adopted by the United States (U.S.) Department of Health & Human Services was used: {0.957×loge [creatinin (mg/dL)]+0.378×loge [bilirubin (mg/dL)]+1.120×loge (INR)+0.643}*10.
For patients on dialysis more than twice a week, creatinine values were always registered at 4.0. All patients were categorized into groups, based on MELD scores: low (<15), moderate (16–25) and high (>25) risk of mortality. The MELD is used as a tool to guide liver organ allocation system for liver transplantation in the US, Europe, and Brazil.30
Statistical analysisData were entered into Microsoft Excel, a spreadsheet program. Frequencies of variables were crosschecked for accuracy. Subsequently, this database was transferred to Epi Info™ 7.1.0.6, through which statistical analyses were performed. Categorical variables (e.g., gender, race/ethnicity, etiology of liver disease) are presented as frequencies and percentages, whereas continuous variables (MELD and CLDQ scores) are presented as means and standard deviations. To evaluate gender differences in average CLDQ scores, a Student's t-test was used, while analysis of variance (ANOVA) was used to compare differences in average CLDQ scores for different categories, level of education, occupation, and family income (p<0.05 was considered significant). Pearson's correlation coefficient was used to verify the existence of correlation between the following variables: CLDQ scores, MELD scores, age, education, and family income.
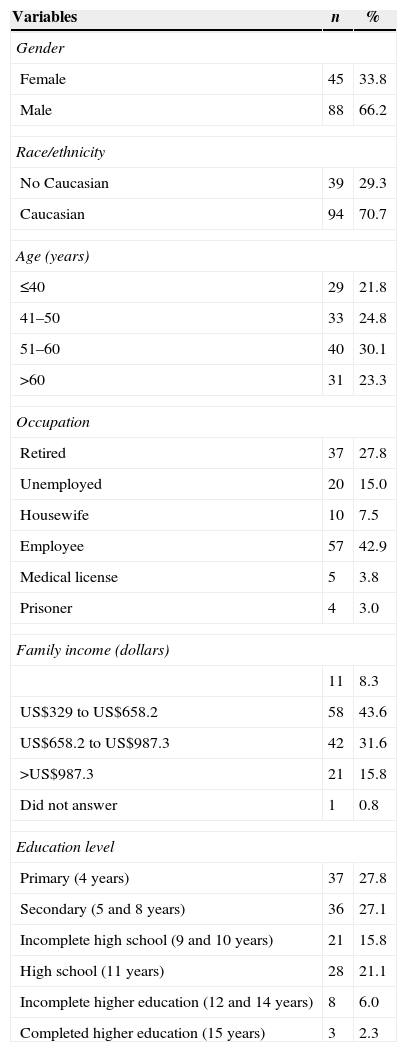
ResultsSociodemographic and clinical characteristicsThe socio-demographic characteristics of 133 patients with chronic liver disease of different etiologies are shown in Table 1. The majority of participants in this study were male (66.2%), Caucasian (70.7%), employed (42.9%), and had a family income of US$329–US$658.2 (43.6%). Over 27% had received up to four years of schooling. The participants’ mean age was 50±13.3 years. Regarding chronic liver etiology, 75 individuals were classified as chronic HCV patients (56.4%), 25 as HBV patients (18.8%), 15 with alcoholic liver cirrhosis (11.3%), 8 with autoimmune hepatitis (6%), 4 with NAFLD (3%), 1 with cryptogenic hepatitis (0.8%), and 5 had other causes of cirrhosis (3.8%).
Socio-demographic characteristics of 133 chronic liver disease patients included in the study.
| Variables | n | % |
|---|---|---|
| Gender | ||
| Female | 45 | 33.8 |
| Male | 88 | 66.2 |
| Race/ethnicity | ||
| No Caucasian | 39 | 29.3 |
| Caucasian | 94 | 70.7 |
| Age (years) | ||
| ≤40 | 29 | 21.8 |
| 41–50 | 33 | 24.8 |
| 51–60 | 40 | 30.1 |
| >60 | 31 | 23.3 |
| Occupation | ||
| Retired | 37 | 27.8 |
| Unemployed | 20 | 15.0 |
| Housewife | 10 | 7.5 |
| Employee | 57 | 42.9 |
| Medical license | 5 | 3.8 |
| Prisoner | 4 | 3.0 |
| Family income (dollars) | ||
| 11 | 8.3 | |
| US$329 to US$658.2 | 58 | 43.6 |
| US$658.2 to US$987.3 | 42 | 31.6 |
| >US$987.3 | 21 | 15.8 |
| Did not answer | 1 | 0.8 |
| Education level | ||
| Primary (4 years) | 37 | 27.8 |
| Secondary (5 and 8 years) | 36 | 27.1 |
| Incomplete high school (9 and 10 years) | 21 | 15.8 |
| High school (11 years) | 28 | 21.1 |
| Incomplete higher education (12 and 14 years) | 8 | 6.0 |
| Completed higher education (15 years) | 3 | 2.3 |
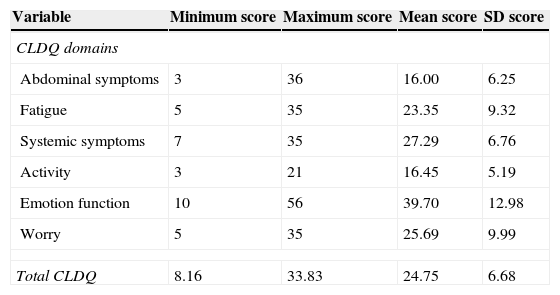
Table 2 shows the minimum, maximum, mean, and standard deviation CLDQ scores. Higher mean CLDQ scores were found for emotional function (39.70±12.98) while lower mean CLDQ scores were obtained for abdominal symptoms (16.00±6.25). Fifty-two patients (39.1%) obtained low (<5) overall CLDQ scores. CLDQ was evaluated according to age, gender, family income and school level. Family income was correlated to CLDQ (r=0.187, p=0.031) (Table 3).
Chronic Liver Disease Questionnaire (CLDQ) score in domains.
| Variable | Minimum score | Maximum score | Mean score | SD score |
|---|---|---|---|---|
| CLDQ domains | ||||
| Abdominal symptoms | 3 | 36 | 16.00 | 6.25 |
| Fatigue | 5 | 35 | 23.35 | 9.32 |
| Systemic symptoms | 7 | 35 | 27.29 | 6.76 |
| Activity | 3 | 21 | 16.45 | 5.19 |
| Emotion function | 10 | 56 | 39.70 | 12.98 |
| Worry | 5 | 35 | 25.69 | 9.99 |
| Total CLDQ | 8.16 | 33.83 | 24.75 | 6.68 |
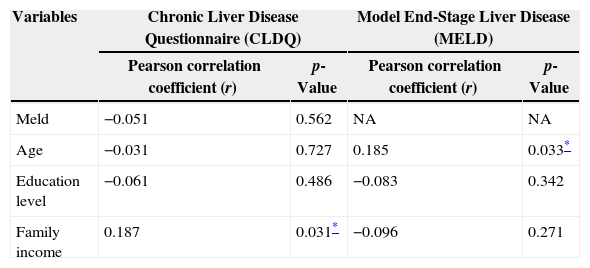
Univariate regression analysis of potential predictors of Chronic Liver Disease Questionnaire (CLQD) and Model End-Stage Liver Disease (MELD) scores, Brazil, 2012.
| Variables | Chronic Liver Disease Questionnaire (CLDQ) | Model End-Stage Liver Disease (MELD) | ||
|---|---|---|---|---|
| Pearson correlation coefficient (r) | p-Value | Pearson correlation coefficient (r) | p-Value | |
| Meld | −0.051 | 0.562 | NA | NA |
| Age | −0.031 | 0.727 | 0.185 | 0.033* |
| Education level | −0.061 | 0.486 | −0.083 | 0.342 |
| Family income | 0.187 | 0.031* | −0.096 | 0.271 |
NA, not applicable.
Regarding MELD, 124 (93.2%) individuals were at low risk (≤15), seven (5.3%) were at moderate risk, and two (1.5%) were at high risk for mortality (>25). MELD indices were correlated to age (r=0.185; p=0.033; Table 3).
DiscussionLiver disease is a serious public health problem that affects patients’ QoL. HRQoL measures are increasingly recognized as important tools for assessing disease outcomes and determining health interventions.17 The present study showed high mean CLDQ scores, indicating low HRQoL in the population studied.
The majority of participants were male, Caucasian, and infected by either hepatitis B or C viruses. This is consistent with other studies using similar methods.31,32 We selected the CLDQ in order to measure the HRQoL of patients with liver disease, regardless of etiology and severity.
Mean emotional functional CLDQ scores were high. The awareness of potentially life-threatening diseases may have adverse emotional effects on participants, regardless of biological consequences of the disease, as suggested by a prior study.10 Previous research has also shown that depressive disorders are related to poor HRQoL in patients with CLD.13
The present study found a positive correlation between CLDQ scores and family income, implying that those with high family incomes had higher HRQoL, as demonstrated by Hsu et al. (2012).33
On the other hand, CLDQ values were not related to age, gender, and level of education. Previous studies have shown that gender and education level were not related to the HRQoL of patients with liver disease.31,34,35 However, CLDQ scores decreased with advancing age; thus, HRQoL also decreased with age.36,37
The MELD index was also used in the present study since it is the main criteria for allocation in liver transplantation waiting list. Most individuals had low MELD indices (scores <15) and were therefore considered to be at low risk of mortality. No correlation was observed between MELD and CLDQ scores. Ray et al. (2010) using CLDQ verified a weak correlation with MELD and concluded that MELD scores poorly predicted QoL, as evidenced in the present study.38 However, a positive correlation was found for MELD scores and age, indicating that as age increases, MELD scores also increase, along with the risk of mortality due to liver disease. Luca et al. (2007) have suggested the inclusion of age into the original MELD formula in order to improve the predictive survival accuracy of patients with cirrhosis.39
Globally, CLD substantially contributes to mortality and morbidity.5,6 Thus, understanding patients’ HRQoL and the extent of debilitation is extremely important. A limited number of studies have been conducted to evaluate HRQoL in patients with liver disease in Brazil, most of which used generic instruments; even so, HCV infection was found to have a substantial impact on patients’ HRQoL.13,40–42
Health, as a social right of all citizens, requires direct and positive state intervention by providing services for diagnosis, treatment, and medical monitoring of diseases including CLD to improve their HRQoL.
This study has some limitations: the cross sectional nature of the study does not allow conclusions about causality; the inclusion of outpatients from one single center; and the necessity of a longitudinal study to determine the true impact on HRQoL in a large population based study.
In conclusion, this study found high mean CLDQ scores among a significant number of CLD patients particularly among those with low family income implying low HRQoL. This suggests that early identification of at-risk individuals with low income could help develop and assess relevant health programs intended to improve patients’ HRQoL. In addition, MELD scores did not significantly predict QoL.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the Coordination of the Improvement of Higher Education Personnel (CAPES), the Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), the Brazilian National Counsel of Technological and Scientific Development (CNPq) for financial support, and Samantha Mucci for providing permission to use the CLDQ questionnaire.