The worldwide elderly population is expected to grow by an additional 694 million people by 2025. By that time, there will be approximately two billion elderly people in the world, most of whom (80%) will be living in developing countries. Based on recent estimates, this population will number over 40 million in 2030 in Brazil and a consequent increase in governmental spending for this population can be expected. Since highly active antiretroviral therapy became available in the mid-1990s, the life expectancy of people living with HIV has increased significantly. Approximately 12 million life years were added to the world between 1996 and 2008 as a consequence of wider access to highly active antiretroviral therapy. In Brazil, the incidence of AIDS among the population aged ≥50 years doubled between 1996 and 2006. The development of antiretroviral therapy has allowed individuals diagnosed at a younger age to live longer, which partially explains the aging tendency associated with the HIV/AIDS epidemic. It is estimated that by 2015, subjects aged ≥50 years will represent 50% of the people living with HIV undergoing clinical treatment. This scenario presents some challenges, including the fact that the diagnosis of HIV tends to be delayed in older patients compared to younger patients because the symptoms of HIV can be confused with those of other common diseases among the elderly and also because healthcare professionals do not consider this population to be at high risk for HIV infection. In regard to the individuals diagnosed with HIV, a further challenge is presented by the morbidity normally associated with aging. Finally, the elderly also exhibit higher susceptibility to the toxic effects and pharmacological interactions of medications. The present article reviews the literature regarding the profile of HIV infection among individuals aged ≥50 years focusing on practical features related to the clinical approach and long-term follow-up of this population.
The worldwide elderly population is expected to grow by an additional 694 million people by 2025. By that time, there will be approximately two billion elderly people in the world, most of whom (80%) will be living in developing countries.1 Brazil has one of the fastest aging populations in the World.2 In half a century (1960–2010), life expectancy of the Brazilian population increased by 25.4 years, having changed from 48.0 to 73.4 years.3 There are currently 15.8 million people aged 60 years or older, and this number is expected to exceed 40 million by 2030.4 It is estimated that the government expenses with this population will increase from 38% to 68% considering the period from 2000 to 2050.5 Since 1998, the availability of drugs for the treatment of erectile dysfunction has extended the length of the active sex life. Moreover, older individuals have a lower tendency to practice safe sex than younger ones.6 This situation may increase the elderly's risk of human immunodeficiency virus (HIV) infection. In addition, the availability of highly active antiretroviral therapy (HAART) since the mid-1990s has significantly increased the life expectancy of people living with HIV (PLH). The worldwide coverage of antiretroviral therapy (ART) increased from 7% in 2003 to 42% in 2008.7 Approximately 12 million life years were added to the world between 1996 and 2008 as a result of the wider access to HAART.7
During the first decade of the acquired immunodeficiency syndrome (AIDS) epidemic, few cases in people aged 50 years or older were reported. However, this number has been increasing steadily.7–11 In Brazil, the incidence of AIDS in this age range doubled between 1996 and 2006.8 The advance of ART has increased the life expectancy of individuals diagnosed at a young age, which partially explains the aging tendency of the epidemic. It is estimated that by 2015, people aged 50 years or older will represent 50% of the PLH undergoing clinical treatment.9 This scenario presents some challenges, including the fact that the diagnosis of HIV tends to be delayed in older individuals, as some symptoms might be confused with those of other common diseases among the elderly and because healthcare professionals do not consider this population to be at high risk of HIV infection. In regard to the individuals already diagnosed with HIV, a further challenge is presented by the morbidity associated with aging. Finally, the elderly also exhibit higher susceptibility to the toxic effects and pharmacological interactions of medications.
The World Health Organization (WHO) and most general practitioners and geriatricians define “elderly” individuals as those aged 60 years or older. With regard to PLH, the US Centers for Disease Control and Prevention (CDC) consider “elderly” individuals to be those aged 50 years or older.12 Being aged ≥50 years is one of the indications to start HAART according to the Brazilian guideline for ART in adults.13 The increased prevalence of HIV among older individuals has not been accompanied by the formulation of therapeutic guidelines or recommendations specific for this population.
The number of older individuals living with HIV/AIDS is increasing, as a result of a growing number of new HIV diagnoses among the elderly as well as the increased survival as a result of better management. Very scarce data are available about elderly individuals with HIV/AIDS in our setting. These data are important not only to serve as guidance for the development of national policies and management guidelines targeting this population but also to guide other low and middle income countries on the several aspects of a reality they will face very soon.
The aim of the present article is to review the literature regarding the profile of HIV infection among individuals aged ≥50 years by focusing on the practical aspects of the clinical approach and long-term follow-up of this population.
Epidemiological dataAccording to the CDC, individuals aged ≥50 years represented 15% of the newly diagnosed HIV cases, 24% of PLH, 29% of people diagnosed with AIDS, and 35% of the deaths caused by AIDS in the USA in 2005.11 In Western Europe, 12.9% of the new HIV cases reported in 2007 involved individuals aged ≥50 years, which is higher than the rate of 3.7% found in Eastern Europe. In Central Europe, the ratio of new cases among the elderly to new cases in younger individuals was approximately 1:10.7
The epidemiological data available for this population in Latin America are limited. In Peru, the HIV/AIDS epidemic mostly affects young people, as 41% of the cases correspond to individuals between the ages of 25 and 34.14 The latest WHO bulletin does not stratify adult cases by age range and does not highlight aging as a specific topic for this epidemic.15
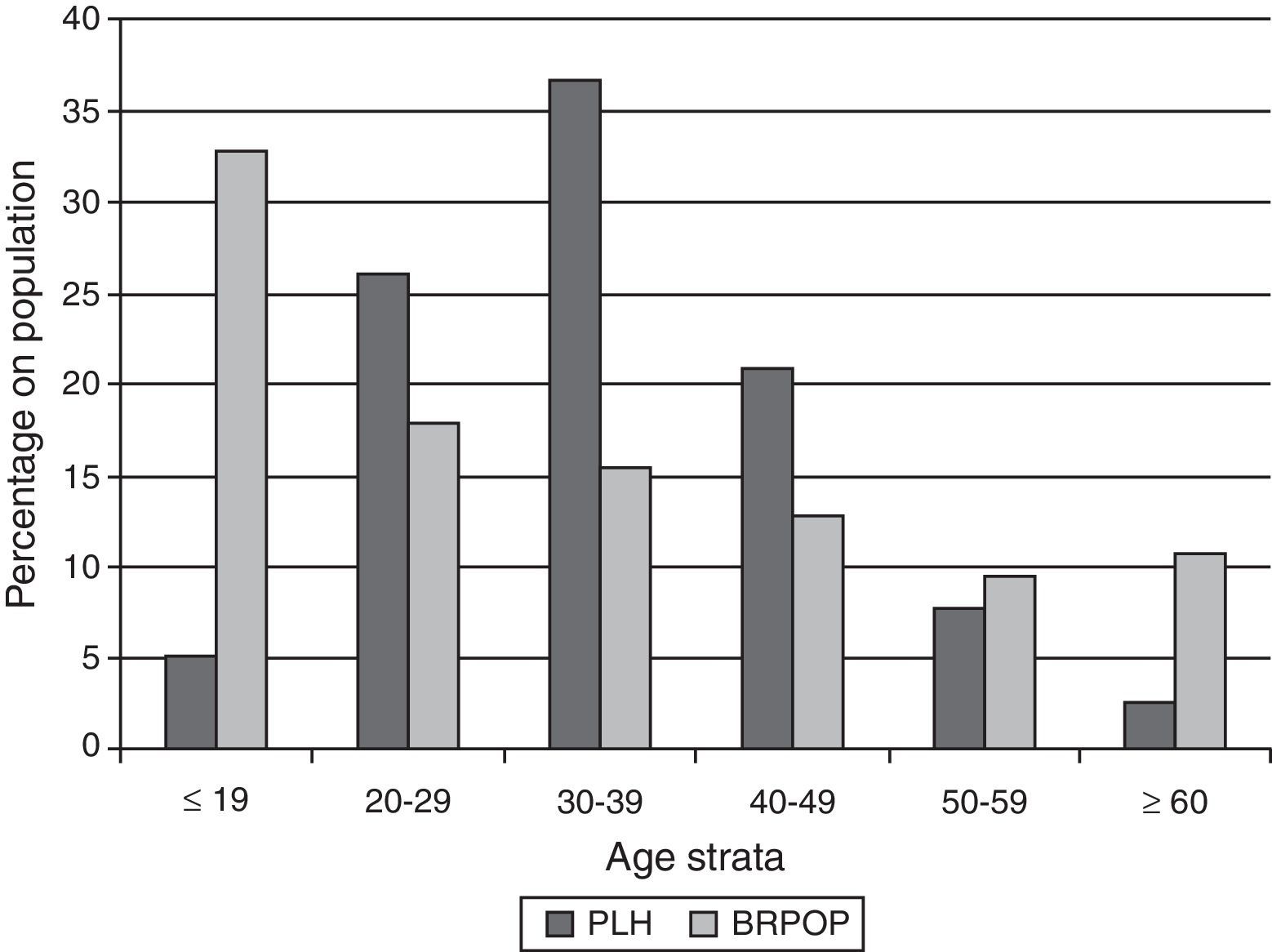
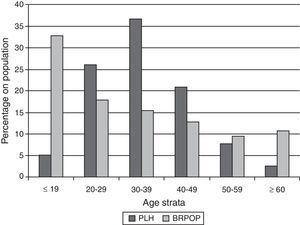
In Brazil, a total of 608,230 cases of AIDS were reported between 1980 and June of 2011; of these, 64,500 (10.6%) corresponded to individuals older than 50 years, most of whom were male (65.0%). The data from this time period show an increase in the AIDS incidence rates among individuals older than 50 years. Among individuals older than 60 years, 10,915 male and 5923 female cases were reported.8 Data provided by the Health Ministry show an increase of 100% in the incidence of AIDS in this population between 2000 and 2010. Comparison of age distribution between PLH (age on AIDS diagnostic) and general population during 2010 in Brazil is depicted in Fig. 1. Percentage of individuals in the Brazilian General Population decreases with age while for PLH in Brazil the majority of cases are diagnosed on age strata between 20 and 49 years.
Comparison of age distribution between people living with HIV-AIDS (PLH) and general population (BRPOP) in Brazil (2010).96
The notion of aging is almost as simple as it is challenging. Over time physical fitness (vision, hearing, and mobility), external appearance (wrinkles and hair loss), and mental agility (efficiency in the retention and processing of new and old information) decline. Although aging is unavoidable, its progression is variable. Performance parameters include the vital capacity of key organs such as the heart, brain, and kidneys. Most body systems exhibit a considerable reserve capacity, such that disease-free aging imposes few constraints on organ function. Generally, organ function is expected to decline by 1% each year beginning at the age of 25.16
The criteria for and definitions of aging vary so widely among geriatricians, researchers, and governmental agencies that there is not even a consensus regarding the “cutoff point” for defining “old age.” For the general population, individuals aged 60–75 years are considered candidates for monitoring/intervention, and this limit falls to 50 years old for PLH.12
Chronological age is the measurement of the years of life since birth. The speed of aging depends on genetic factors, whereas environmental, biological, and lifestyle-related factors also influence the biological or phenotypic age. Senescence, i.e., the increase in the chronological age of a population of predominantly indivisible cells in culture, is an essentially biological definition of aging and is considered to be a natural process. In contrast, senility corresponds to aging under pathological circumstances.
Genetic factors – the role of telomeresAll chromosomes of eukaryotic cells include a structure known as the telomere (from the Greek, telos, end, and meros, part), which is a marker of cellular division. Telomeres are a kind of “cap” that protect the ends of chromosomes and consist of proteins and noncoding DNA. Their function is to maintain the structural stability of chromosomes. Under normal aging conditions, the telomeres shorten at each cell division until the cell totally or partially loses its ability to divide, leading to disease and death. Telomeres play the role of a biological clock. The synthesis of telomeres occurs at the end of DNA replication by the action of the enzyme telomerase, which is a reverse transcriptase, and telomerase activity depends on the activation of a specific gene that is not activated (“turned on”) in all cells.
The role of telomeres in CD4+ T-cell depletion and in clonal replication of these cells during HIV infection was described in 1966.17 In HIV+ individuals, the length of telomeres is similar to that of non-infected elderly individuals, which might indicate a predisposition to premature aging. This finding might explain the presence of abnormalities common to the elderly in HIV+ younger individuals, such as low numbers of CD4+ T cells and decreased thymus activity. The physiopathology of aging in PLH with or without prolonged ART is still poorly understood. The use of thymidine analogs appears to induce telomere shortening.18
The oxidative stress that occurs in natural aging seems to favor the replication of HIV, and the presence of HIV, in turn, appears to accelerate the natural aging process. Factors involved in the progressive loss of the vital (reserve) capacity of the bodily systems in PLH, leading to organ failure, reduced cognitive functions, disease, and death include: the presence of comorbidities; viral hepatitis coinfection; alcohol abuse, tobacco, or other drugs; microbial translocation; and chronic immune dysfunction, in addition to the toxicity of ARV agents and other drugs used concomitantly.19
Effects of HIV infection and aging on immunityBy itself, aging is associated with complex changes in the immune system that increase susceptibility to infections, autoimmune and neoplastic diseases, and reduce the response to active immunization.20 During the natural progression of HIV infection, the immune system is chronically and progressively impaired. HIV infection and normal aging exert many similar effects on the immune system. For example, the T-cell population, particularly in the gastrointestinal tract, is strongly affected by both HIV and aging, as are nearly all aspects of immunity.21
B cells and antibody productionAging and HIV infection can also affect immunological memory. HIV infection is associated with an increased activation of naïve B cells, which persists even during the use of HAART.21 Some examples of the B-cell dysfunction that occurs in both PLH and older adults include the increased risk of severe bacterial infections with pathogens such as Streptococcus pneumoniae and the reduced ability of polysaccharide antigens to activate B cells, demonstrated by a poor response to the pneumococcal vaccine in terms of the production of efficacious antibodies and clinical protection.22
T-cell functionThe number of naïve T cells (CD4+ and CD8+) decreases with age and HIV infection, and this can also influence the effect of ART.22 The number of such cells is as low in PLH as in non-infected individuals aged 20–30 years older. The T cells become less responsive, are less able to proliferate, and exhibit alterations in the signaling receptors and surface markers, including a loss of CD28 expression.23 These changes result in an increase of the Th-2 cells and simultaneous decrease of the Th-1 cells. Consequently, the levels of interleukin-2 (IL-2), which is produced by Th-1 cells, decreases as a result of aging; this phenomenon represents one of the most common cytokine alterations observed in immunosenescence.
The number of cytotoxic CD8+ T lymphocytes (CTL) increases in HIV infection in both young and old individuals.24 The memory T cells express surface CD28, which stimulates cell division in the presence of antigens. “Old” memory cells tend to lose CD28 expression and thus multiply less than younger cells upon antigen exposure.25
During chronic HIV infection, the repeated expansion of the CD8+ T cell population leads to a loss of CD27 and CD28 by the T cells, resulting in a predominance of lymphocytes that lack the receptors needed for co-stimulation and efficient antigen presentation. Eventually, the cells attain replicative senescence (exhaustion), which is characterized by shorter telomeres, low telomerase activity, and increased production of pro-inflammatory cytokines. A high proportion of CD8+ CD28 CTL is predictive of early mortality19 and rapid progression to AIDS among PLH,23 although a causal relationship has not yet been established.
The immune function of the mucous membranes is severely impaired by HIV infection. Independently of the route of infection, HIV replicates more intensively and the CD4+ T cell population is exhausted much more quickly in the gut-associated lymphoid tissue (GALT) than in the peripheral blood. This reduction in the number of CD4+ T cells in the GALT does not recover after the onset of HAART as it does in the peripheral blood. The effects of aging on the GALT are still poorly understood. The consequences of T-cell senescence are profound, and the ability to control chronic viral infections is lost.
Age and risk of HIV infectionNew cases of HIV infection among individuals aged ≥50 years have been reported. The CDC estimated that approximately 7000 new cases of HIV infection occurred in this population in the USA in 2009.11 According to an American report with 3000 individuals aged 57–85 years, many older people are sexually active on a regular basis (73%, 53% and 26% for 57–64, 65–74 and 75–85 years, respectively).26 In another study conducted in the US, 20–30% of men and women in their 80s reported being sexually active.27 Therefore, risky sexual behaviors of individuals ≥50 years of age must be addressed. In a study conducted in London (UK) older HIV+ MSM (men who have sex with men) are just as likely to report unsafe sex as younger HIV+ MSM.28 In contrast, a study conducted in the US with HIV+ women verified that condom use decreased with age while lubricant use increased with age.29 Older individuals, particularly heterosexuals, might not have an accurate understanding of their risk for HIV, most likely as a function of the typical features of AIDS at the onset of the epidemic, when AIDS occurred mostly in young homosexual men. Many heterosexual individuals ≥50 years are single, have no stable partners, are divorced or widowed, and have active sexual lives, often involving partners who are also unaware of the need to practice safe sex. There seems to be reluctance on the part of both older heterosexual men and women to use condoms. Certainly, unwanted pregnancy is not a concern among individuals of this age range, and men with erectile dysfunction might be particularly unlikely to accept condoms.26 Biological mucosa modifications subsequent to low estrogen levels causing decreased lubrication might favor the production of mucosal lesions that increase the risk of HIV transmission. Similarly, biological changes in the anal mucosa make it susceptible to lesions that also facilitate the transmission of HIV.30 The strategies for behavioral interventions aimed at promoting the use of condoms among the elderly population must be reassessed. In addition, more recently investigated preventive strategies, such as the treatment of positive partners of serodiscordant couples and pre-exposure (PrEP) and post-exposure (PEP) prophylaxis, need to be assessed because this population might have less access to or may not be targeted for such preventive strategies.
Life expectancy and HIV infectionSurvival estimates for PLH in the period corresponding to the introduction of HAART (1996–2005) were approximately two-thirds of the life expectancy of the general population.31 Since that time, there have been advances in treatment, including wider access to ART and increased knowledge regarding issues such as prevention of cardiovascular disease (CVD) and control of metabolic diseases in HIV+ individuals. Life expectancy of HIV+ individuals is influenced by social and demographic factors in addition to their level of immunosuppression. For example, the time the CD4+ T-cell count remained below 100cells/mm3 might be an important predictor of death.32
Nevertheless, the current life expectancy of patients starting HAART early and thus achieving normal CD4+ counts sooner might be very similar to that of the general population.33
HAART and agingTwo different scenarios must be taken into account with regard to HAART and aging. One situation involves HIV diagnosis in an individual aged ≥50 years, and the other involves individuals who have lived with HIV for many years (chronic cases) with complications typically not expected for a given age range. PLH include those of all age ranges and may therefore be exposed to different durations of infection and different degrees of toxicity associated with long-term use of ART. Therefore, the impact of ART among HIV+ patients starting treatment after age 50 must be thoroughly understood in addition to the impact of ARV drugs on the cardiovascular and metabolic disorders that can be expected in aging people with HIV. Interruptions or modification of ARV regimens are often needed in such patients to minimize drug interactions or toxicity. In addition, more thorough or frequent assessments might be needed in the surveillance of associated morbidities and preventive interventions during the follow-up care of elderly individuals with HIV.
Clinical outcomesIn general, the clinical manifestations of immunodeficiency appear late in the course of HIV infection. However, in most cases, diagnosis is established only after the immune system has been strongly affected and CD4+ T-cell count is less than 200cells/mm3. Older people are diagnosed at more advanced stages of HIV infection than younger individuals.34 This delayed diagnosis might partially explain some studies’ findings of poorer clinical outcomes, including shorter intervals between the identification of HIV, the AIDS diagnosis and reduced length of survival,7 in elderly patients than in younger patients. Healthcare professionals do not seem overly concerned with the possibility of HIV infection in older individuals, possibly because older people are less likely than younger people to be considered sexually active or intravenous drug users. In addition, many of the symptoms of HIV infection might be confused with signs of natural aging, such as weight loss, fatigue, and cognitive or visual problems.35 The older patients themselves also may not suspect of their HIV infection. Most individuals diagnosed with HIV after age 60 never suspected that they were infected before they were tested.36
Studies conducted in the pre-HAART era found an association between old age and the risk of death among HIV infected individuals.37 At the beginning of the HAART era, access to ART was identified as the only factor associated with survival in patients older than 50 years.38 Improvement of ART efficacy has been correlated with longer life expectancy among individuals with HIV infection, and it is estimated that 50% of the currently treated population will be aged ≥60 years in 2015.39
Early mortalityEarly mortality risk after the HIV infection diagnosis increases with age. A CDC survey on 12-, 24-, and 36-month survival after diagnosis showed that mortality was substantially higher among older patients at all three time points assessed.40 A study comparing early mortality between the cities of Rio de Janeiro and Baltimore also found a higher risk of death among the elderly (hazard ratio (HR) 1.03; p=0.03).41
Immune response to HAART and viral suppressionIn terms of the CD4+ T cell count improvement and viral suppression at different age ranges, most studies found that older people can achieve success rates similar to younger individuals given adherence to HAART.42,43 Greenbaum et al. found that the average time to attain the first undetectable viral load (VL) was lower among patients aged ≥50 years than patients aged ≤40 years (3.2 vs. 4.4 months; p=0.001). This difference was more significant when HAART included one protease inhibitor (PI).44
Nevertheless, the immune recovery rate can be slower and less robust among older patients. Data from the NA-ACCORD cohort study showed that the probability of recovering the CD4+ T-cell count (an increase of at least 100cells/mm3 over the first two years of ART) decreased with age.45 Low treatment compliance does not seem to explain these outcomes because older patients exhibit better adherence than younger patients and have a lower risk of viral rebound.46
Selection of ART regimensIt is recommended that individual ART regimens take the patient's lifestyle, among other factors, into account. Age is also an important factor for the selection of ART. Nucleoside analogs are strongly associated with mitochondrial toxicity, and zidovudine (ZDV) causes bone marrow suppression. Older patients might exhibit reduced renal function and loss of bone mass in addition to depression and cognitive disorders. There is more information regarding aging patients with HIV infection that have been exposed to ART for long periods of time than information regarding how to treat an old HIV+ patient recently diagnosed. Moreover, the choice to start treatment for individuals who have a diagnosis in the age groups above 50–60 years is probably something new for most clinicians. As the proportion of older patients starting treatment increases, studies assessing the efficacy and tolerability of regimens for this particular population are needed.
Tolerability to ARTDue to greater need for concomitant medications in older patients, elderly HIV+ patients are expected to have poorer tolerability to ART, higher toxicity, and more numerous drug interactions. However, there are currently few data regarding these aspects.47 In the Swiss cohort, older patients tended to use more concomitant medications than younger patients and also exhibited higher risks of drug interactions, which were mostly related to the effects of ART on the action of the concomitant medications. Toxic effects most commonly described are associated with gastrointestinal intolerance, followed by central nervous system disorders, hepatotoxicity, and dyslipidemia.48
There is little information regarding the reasons to change ART in the elderly population. Among 95 patients aged ≥50 years in the IPEC/Fiocruz cohort starting their first HAART regimen between January 1996 and December 2008, 59% modified or discontinued (MOD) the first regimen (28.1/100 individuals per year of follow-up); the mean time to MOD was 12 months, and the main reasons for MOD were therapeutic failure (14%), toxicity during the first year of HAART (11%), and long-term toxicity (6%). The incidence of MOD was significantly higher among patients who started HAART with CD4+ T-cell counts<100cells/mm3 (HR 1.97; 95% confidence interval (CI) 1.08–3.61).49 In a German cohort, a change of regimen was more common among patients aged ≥50 years.50
Adherence, resistance and drug interactionsSome studies found higher adherence to ART among patients aged ≥50 years compared to younger patients.46,51
Data regarding the resistance profile in therapeutic failure among elderly patients are scarce, as are data on primary resistance and age. In a German cohort, no difference was found in transmission of resistance between older and younger patients.50 In an Italian cohort, increased age was independently associated with higher likelihood of primary resistance.52
Older patients tend to have more comorbidities, fall ill more often, and pharmacological intervention is frequently needed, with a consequent high risk of drug interactions. Older HIV+ patients use more medications for CVD, gastrointestinal, and hormonal problems than younger patients, whose use of these medications matches that of the general population.48 In the Swiss cohort, age was one of the risk factors associated with increased drug interactions.53
Non-AIDS-defining illnesses associated with HIVHAART reduced AIDS-related morbidity and mortality; however, the death rate among HIV+ individuals attributed to non-AIDS-defining illnesses, including liver, lung, cardiovascular, and neoplastic diseases, increased.54 Many such illnesses correlate with HIV after adjustment for well-established demographic and behavioral risk factors. Some examples include myocardial infarction, thrombosis, and stroke; bone diseases, including osteoporosis and avascular necrosis; neoplasias caused by infectious agents such as human papillomavirus (HPV)-related anal cancer; and neoplasias not caused by infectious agents, such as lung cancer; chronic obstructive pulmonary disease, dementia, liver fibrosis progressing into cirrhosis, and hepatocarcinoma; hematologic diseases such as anemia and thrombocytopenia; and kidney dysfunction, including end-stage failure.54
Chronic immunosuppression has been associated with many such conditions in large cohort studies, where low CD4+ T-cell counts are predictive of death by non-AIDS-defining outcomes. The incidence of such conditions is higher among people aged ≥50 years.23
Some authors have disputed the correlation between HIV/HAART and the early appearance of comorbidities based on the predominance of retrospective analyses among these studies and the lack of sufficient data regarding well-established risk factors such as smoking, abuse of alcohol and recreational drugs, socioeconomic status, and concomitant medications.55
MalignanciesThe higher incidence of malignancies in PLH is related to the chronic oncogenic effect of some viral infections but is not necessarily related to aging. Nevertheless, some important aspects related to HIV, age, and the incidence of neoplasias are still controversial, such as whether HIV leads to early occurrence of cancer, whether the high incidence of some malignancies among PLH is a consequence of accelerated aging, and whether all patients should be treated with HAART upon diagnosis of HIV infection to achieve steady viral suppression and maintain immunity close to its normal status. However, it is unclear whether avoiding severe immunodeficiency alone reduces the risk of HIV-related malignancies.56
Predictive factors of non-AIDS-defining malignancies include age (the risk rate doubles per ten years of age increase); the most recent low CD4+ T-cell count; a history of smoking, and hepatitis B (HBV) coinfection.57 HAART is protective against AIDS-defining malignancies but does not seem to protect against non-AIDS-defining malignancies.57 Some studies have shown that since HAART was introduced, non-AIDS-defining malignancies have been diagnosed earlier in HIV/AIDS patients.57,58 PLH might also be at a higher risk of cancer due to peculiarities of their lifestyle, particularly smoking and use of alcohol.56
As the HIV+ population ages while exhibiting persistent alterations of the immune system, an increasing number of malignancies can be expected over time. Lung, anal, and liver cancer, as well as Hodgkin's lymphoma, represent the main contributions to this increase. In the US, the incidence of breast, colon, and prostate cancer is lower among HIV+ individuals than among the general population; this may be because the HIV+ individuals had not reached a sufficient age to develop such malignancies or to some protective effect related to HIV.56
In any case, the prevention and early treatment of neoplasias in PLH must be included in public health follow-up strategies and therapeutic guidelines targeting the elderly population.
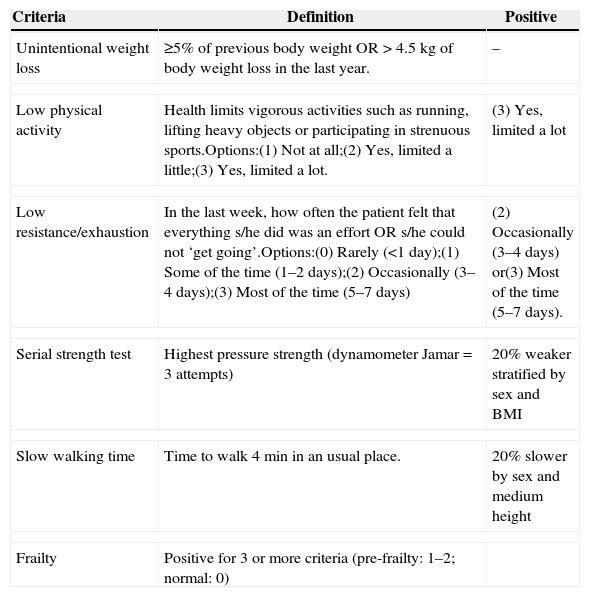
FrailtyFrailty is a pathologic aging syndrome, the mechanism of which is unknown, that leads to unfavorable outcomes such as hospitalization, disability, and death.59 Increased free radical levels, mitochondrial dysfunction, and cytokines might activate inflammatory pathways, leading to this condition. The levels of C-reactive protein, d-dimer, fibrinogen, and IL-6 are increased in older individuals with the frailty phenotype.60 Similarly, HIV infection and ART toxicity activate the inflammatory mechanisms associated with frailty.59 There is no universal definition of “frailty” for patients with HIV/AIDS; in the general population, a frailty phenotype is established when at least three of the following traits are present: exhaustion, slow walking speed, low levels of physical activity, weakness, and weight loss.60 HIV infection seems to accelerate the development of frailty, even when the patient exhibits viral suppression under HAART. In addition to HIV, frailty has also been associated with age, the female gender, a low level of schooling, being single, abuse of non-prescription drugs, and depressive symptoms. Table 1 describes an algorithm for the calculation of frailty that can be applied to patients.
Frailty calculation.81,82
| Criteria | Definition | Positive |
|---|---|---|
| Unintentional weight loss | ≥5% of previous body weight OR>4.5kg of body weight loss in the last year. | – |
| Low physical activity | Health limits vigorous activities such as running, lifting heavy objects or participating in strenuous sports.Options:(1) Not at all;(2) Yes, limited a little;(3) Yes, limited a lot. | (3) Yes, limited a lot |
| Low resistance/exhaustion | In the last week, how often the patient felt that everything s/he did was an effort OR s/he could not ‘get going’.Options:(0) Rarely (<1 day);(1) Some of the time (1–2 days);(2) Occasionally (3–4 days);(3) Most of the time (5–7 days) | (2) Occasionally (3–4 days) or(3) Most of the time (5–7 days). |
| Serial strength test | Highest pressure strength (dynamometer Jamar=3 attempts) | 20% weaker stratified by sex and BMI |
| Slow walking time | Time to walk 4min in an usual place. | 20% slower by sex and medium height |
| Frailty | Positive for 3 or more criteria (pre-frailty: 1–2; normal: 0) | |
HIV infection is associated with a higher risk of metabolic disorders such as insulin resistance, pro-atherogenic lipid profiles, and alterations of the subcutaneous and visceral body fat distribution (lipodystrophy). Such disorders might contribute to the higher risk of CVD associated with HIV and ART.61 Some cross-sectional studies have found an increased risk of metabolic and hormonal disorders, including osteopenia, hypogonadism, diabetes mellitus, and dyslipidemia.62,63 In the Swiss cohort, a higher proportion of non-AIDS comorbidities and multiple morbidities such as diabetes mellitus and CVD were observed among older individuals.64
The earliest cohort studies associated HIV infection and exposure to ART with a risk of coronary disease and myocardial infarction (MI). Subsequently, Friis-Moller et al. found that the risk of MI was more strongly associated with pre-established risk factors such as advanced age.65 Nevertheless, the correlation among HIV infection, inflammation, and cardiovascular risk has not yet been fully elucidated.66 HIV infection, prolonged ART, and aging are known to contribute to lipoatrophy and metabolic disorders.67 In the American VACS cohort, the risk of MI was twice as high among the HIV+ than the non-infected individuals, even after adjusting for established risk factors such as age, race, hypertension, diabetes mellitus, hypercholesterolemia, smoking, infection with hepatitis C virus (HCV), kidney disease, body mass index (BMI), and history of cocaine and alcohol abuse. However, the age at which MI occurred did not differ significantly among the older individuals, regardless of HIV status.68
Renal functionRenal function is reduced in both elderly and PLH, leading to an impaired elimination of drugs and an increased risk of toxicity and mortality associated with CVD.69 Although the survival of PLH with end-stage kidney disease has improved since the introduction of HAART, its incidence has not changed.69 The survival rates of elderly patients undergoing dialysis are low, and a delay in the initiation of dialysis is associated with even greater morbidity and mortality. It is not yet known whether the control of arterial pressure and glycemia might delay the progression of kidney disease in HIV+ patients as it does in the general population.69
Liver diseaseLiver disease is an important cause of death among PLH, surpassed only by opportunistic infections. Morbidity and mortality due to liver disease are up to 4-fold higher among the elderly than among young adults. In addition, the liver volume, blood flow, drug metabolism, and regenerative capacity decrease with age. Age is also associated with greater risk of hepatocellular carcinoma. The progression of chronic hepatitis C into cirrhosis is accelerated in HIV+ patients.70
Cognitive impairment and HIVThe natural history of HIV infection is changing, and factors associated with advanced age will become an important modulator of its clinical outcomes, particularly dementia. Historically, there was no need to consider the potential contribution of age-related neurodegenerative diseases such as Alzheimer's to cognitive disorders among PLH because HIV primarily affected young individuals, whose life expectancy was then low. With the advance of HAART, there is an increased likelihood that young and middle-aged HIV+ individuals will develop premature neurodegenerative disorders that could directly impact their ability to work and reduce their quality of life.71 Increased longevity under conditions of chronic HIV-mediated inflammation combined with the secondary effects of HAART might significantly contribute to the increased risk of early cerebral aging and cognitive loss. Although the incidence of HIV-related dementia decreased after HAART was introduced, several studies reported a correlation between old age and an increased risk of HIV-associated dementia as the initial AIDS-defining illness.32
SmokingThe well-established risks of smoking are considered greater among PLH, as these risks may be enhanced by the chronic inflammation associated with HIV, even among individuals with appropriate viral suppression. Associated questions include whether PLH smokers are at a higher risk of lung cancer or other malignancies as well as cardiovascular complications compared to smokers in the general population. As PLH are aging, their risk for all age-related diseases, including malignancies, will increase. The impact of being a smoker among HIV+ individuals who are aging needs to be established, once the prevalence of smokers among PLH is high, ranging from 50 to 70%.72
Vitamin D deficiencyAlthough the rate of vitamin D deficiency is high among PLH73,74 little is known about the benefits of vitamin D supplements in metabolic disorders other than those involving the bones. The available data are controversial and non-standardized. In addition, the ideal replacement and maintenance regimes for PLH are not known. It is also important to highlight that bone diseases, depression and cognitive impairments can be associated with both aging and vitamin D deficiency. Therefore, controlled trials assessing both the success of vitamin D replacement and its benefits are needed.
Bone mass lossHIV causes chronic activation of T cells and increases the production of pro-inflammatory cytokines, which increase the activity of osteoclasts, and these alterations persist despite effective ART and viral suppression.75 The current understanding of bone metabolism in the aging HIV/AIDS population is limited, and the sparse longitudinal data available show conflicting results.76 Despite the currently limited understanding of the factors associated with bone mineral reduction, the assessment of risk factors for bone loss is needed to reduce the morbidity of osteopenia and osteoporosis in the HIV+ aging population.
Women with HIV/AIDS and menopauseA study conducted in Germany found that women reported more age-related complaints (such as mood swings and impaired mobility) than men, and 80% of the women believed that they were aging prematurely, compared to 18% of men (p<0.001).77 Early menopause seems to be related to HIV-induced immunodeficiency and is more common among women with low CD4+ T-cell counts. Risk factors described and established for the general female population, such as African descent and a history of intravenous drug use, have been associated with early menopause among HIV+ women.78 In turn, early menopause influences the overall health of women and might be associated with reduced sexual function and depressive symptoms in addition to increased cardiovascular risk and skeletal health issues. Recently, higher rate of bone mass loss was found in the spine and forearms of postmenopausal HIV+ women compared to non-infected women, independent of stable ART.79 The loss of bone mass is likely to increase the risk of fractures in this population. Therefore, stratification of risk by means of bone densitometry and research on secondary causes of osteoporosis and its appropriate treatment in all postmenopausal HIV+ women seems justified. The usefulness of such assessments in younger women has not yet been established.
Routine measures that should be incorporated into the preventive care and health maintenance of older individuals living with HIV/AIDSRegular physical exercise is extremely important to the quality of life of PLH, especially older individuals. A study performed in São Paulo, Brazil, found that resistance training increased the strength of older patients and allowed them to achieve the same level of performance as the healthy control patients, even when their initial clinical profile was worse.80
In addition to physical exercise, other healthy habits such as eating a balanced diet; controlling the blood pressure; treating diabetes and dyslipidemia; and abstaining from smoking, alcohol, and drugs, must be strongly encouraged in PLH, particularly among the older. Routine measures applied to the general population that should be incorporated into the preventive care and health maintenance of PLH are described in Table 2.
Routine measures used in the general population that must be incorporated into the preventive care and health maintenance of individuals living with HIV/AIDS (PLH).83–95
| Comorbidity | Risk factors | Preventive measures |
|---|---|---|
| Anal cancer | • Infection by human papillomavirus (HPV) | • The anti-HPV recommendations are currently the same as those established for the general population and might have a future impact; however, recommendations specifically for the HIV-infected population are still being established, and several strategies are currently being studied. Screening for anal cancer prevents invasive cancer by means of the identification and removal of precancerous lesions (high-grade anal intraepithelial neoplasia – HGAIN) before their progression. Initial screening tests usually include a digital rectal examination, visual inspection, and anal cytology. When abnormal, patients are referred for high-resolution anoscopy and biopsy of lesions suspected of HGAIN. HGAIN areas are removed by ablation to reduce the risk of progression into anal cancer. Cost-effectiveness studies of these strategies are currently being conducted. |
| Arterial hypertension | • Gender: until menopause, women are more protected• Race: Afro-descendants exhibit higher risk than Caucasians• Familial history• Sedentarism• Obesity• Contraceptives (women aged>35 years)• Smoking• Use of alcohol• Nutritional habits (high salt consumption)• Low socioeconomic level | • Pre-hypertension (systolic arterial pressure – SAP=120–139mmHg or diastolic arterial pressure – DAP=80–89mmHg): Increased attention to lifestyle habits such as eating a healthy diet and exercising regularly.• Hypertension stage I (SAP=140–159mmHg or DAP=90–99mmHg): thiazide diuretics. Also consider the use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, calcium-channel blockers, or a combination of these.• Hypertension stage II (SAP≥160mmHg or DAP≥100mmHg): combination of 2 anti-hypertensive agents |
| Breast cancer | • Personal or familial history of breast cancer• No children• Significant exposure to X-rays• Early menarche• Late menopause• High socioeconomic class• First pregnancy after age 30• Fat-rich diet• Prolonged use of oral contraception (disputed) | • Clinical and self-examinations• Refer to gynecologist for periodical assessment• Mammography• Ultrasound |
| Cancer of the respiratory system (lung, throat, larynx) | • Familial history• Smoking | • Lung cancer screening; early assessment when symptoms are present• Smoking cessation• Preliminary data suggest that thorax computed tomography with low radiation doses might be beneficial, but further studies are needed. |
| Cardiovascular disease and stroke | • Smoking• Sedentarism• Dyslipidemia• Menopause (women)• Glucose intolerance• Familial history• Obesity (BMI>30kg/m2)• Abdominal obesity (men>94cm, women>80cm)• Metabolic syndrome• HIV infection appeared as an independent risk factor; however, risk increases when CD4+ count is low and VL is persistently high (loss of virologic control). | • 1st step – assess CVD risk: Framingham scale• Use of aspirin (dose according to coronary disease risk calculated by Framingham score)• Control of blood pressure• Control of cholesterol• Smoking cessation• Control of diabetes and prediabetes• Aerobic physical exercise (30min, 3–5 days/week)• Balanced diet to maintain healthy body weight: fruits, vegetables, whole grains, fiber-rich foodstuffs, fish (especially oily fish twice a week)• Restrict saturated fat to <7% of daily intake, trans fat to 1%, and cholesterol to <300mg/day.• Reduce consumption of sodas and foodstuffs containing added sugar.• Choose and prepare foodstuffs with little salt.• Use alcohol moderately. |
| Colon/bowel cancer | • Familial history of colorectal cancer• Diet high in fat and meat and low in calcium• Obesity• Sedentarism• Inflammatory bowel disease | • Colonoscopy; screening starting at age 50 is recommended for the general population• Smoking cessation |
| Dementia/Alzheimer's disease | • Familial history | • Cognitive exercises• Dementia after stroke: lifestyle changes, including regular physical exercise, a balanced diet, and the control of high blood pressure and diabetes mellitus• Alzheimer's disease: no prevention is available |
| Diabetes mellitusNormal glycemia: <100mg/dL fasting or <140mg/dL 2hours after intake of 75g of dextrosePrediabetes: 100–120mg/dL fasting or 140–200mg/dL after dextroseDiabetes Mellitus: fasting glycemia≥126mg/dL, >200mg/dL after dextrose or casual glycemia, or >200mg/dL when symptoms are present | • Age equal to or older than 45 years (HIV non-infected individuals)• Familial history• Sedentarism• Low high-density lipoprotein (HDL-c) or increased triglyceride levels• Arterial hypertensionCoronary disease• Previous gestational diabetes• Children with birth weights higher than 4kg, repetitive abortions, children dying during the first days of life• Use of medications that increase glucose (cortisone, thiazide diuretics, beta-blockers)• HIV-infected patients using ART exhibit a fourfold higher risk. | • Diet control/planning• Physical exercise• Weight loss• Restricted use of alcohol• Glycemic control• Smoking cessation• Avoid pancreas-damaging medications (cortisone, thiazide diuretics).• Pharmacological treatment: metformin is the first choice and might be associated with sulfonylureas• Insulin might be needed as an adjuvant of oral drugs or as a second choice because it improves insulin resistance and has possible effects on the lipids and body composition. Risk of hypoglycemia• More complex cases must be referred to specialists. |
| Dyslipidemia | Types of dyslipidemia:• Isolated hypercholesterolemia: low-density lipoprotein – LDL-C≥160mg/dL• Isolated hypertriglyceridemia: TG≥150mg/dL• Mixed hyperlipidemia: LDL-C≥160mg/dL and TG≥150mg/dL simultaneously• Low HDL: isolated reduction of HDL-C (<40mg/dL in males and <50mg/dL in females) or associated with increased LDL-C or TG | • Changes in lifestyle: regular physical exercise, weight loss, smoking cessation, nutritional therapy• Isolated hypercholesterolemia: statins (LDL-C reduction; lower TG reduction; slight HDL-C increase); ezetimibe (combined with a statin). Use cautiously in patients using ART due to the risk of interactions. Simvastatin and lovastatin cannot be used due to interaction with ART.• Mixed hyperlipidemia (increased cholesterol, LDL-C and TG): fibrates (bezafibrate, ciprofibrate, fenofibrate, and gemfibrozil). Caution is needed due to interaction with ART; nicotinic acid; omega-3 fatty acids.• Elderly: attention to secondary causes of dyslipidemia, mainly hypothyroidism, diabetes mellitus, and chronic kidney failure |
| Hepatitis A (HAV) | Intake of contaminated waterLack of basic sanitation | • Personal hygiene (intake of treated water, washing hands, avoiding foodstuffs of unknown origin)• Basic sanitation: (sewage, septic tanks)Laboratory test for HAV markers (anti-HAV IgM and anti-HAV IgG)• Vaccination: schedule for susceptible individuals (non-reagent HAV IgG serology) – 2 doses (at 0 and 6–12 months or 0 and 6–18 months). Not included in the Handbook for CRIE – Health Ministry (Brazil)• Lower response to vaccine in individuals with CD4+<200 |
| Hepatitis B (HBV) | • Multiple sexual partners• Healthcare professionals• Patients undergoing hemodialysis• Injection drug use | • Use of condoms• Universal precautions with biological materials• Laboratory tests for HBV markers (HBsAg, anti-HBs, anti-HBc IgM, anti-HBc)Vaccination: 4 double-dose applications (at 0, 1, 2, and 6 months)• Butantan (Brazil): Up to 18 years old: 1mL; ≥ 19 years old: 2mL• Lower response to vaccine in cases of advanced immunodeficiency, patients with detectable viral load, and those with transiently increased viral load. Measurement of anti-HBs is recommended (4–6 weeks after the last vaccine dose) because revaccination might be needed. |
| Hepatitis C (HCV) | • Multiple sexual partners• Injection drug and inhalants use | • Use of condoms• Universal precautions with biological materials• Not sharing needles and other tools used in the preparation and consumption of injectable drugs and inhalants (straws)• Laboratory tests for HCV markers (anti-HCV) |
| Liver cancer | • Alcoholism• HCV and HBV coinfection | • Careful monitoring of patients with chronic HBV and HCV infection; treatment when indicated, periodic assessment of the liver function, viral load, and possibly alpha-fetoprotein |
| Menopause/andropause | – | Hormonal replacement |
| Metabolic syndrome | Characterized by• Glucose≥100mg/dL• Triglycerides≥150mg/dL• Blood pressure≥130/85mmHg• HDL cholesterol<50mg/dL (men) or <40mg/dL (women)• Abdominal circumference>80cm (women) and >94cm (men) | • Diet• Regular physical exercise• Lipid profile• BMI reduction to 18.5–24.9kg/m2 |
| Osteopenia/osteoporosis | • Female gender (menopause)• Smoking• Low calcium intake• Vitamin D deficiency• Vitamin A excess• Sedentarism• High caffeine intake• High salt consumption• Immobilization• Falls• Alcohol (more than 3 drinks/day)• Low BMI• Osteoporosis is diagnosed by means of BMD measurements using dual-energy X-ray absorptiometry (DXA) of the hips and lumbar spine. The measurements are expressed as g/cm2 for the same age and gender (Z score) and for same-gender young adults (T score). The WHO defines osteoporosis as a T score≤−2.5, which does not apply to women before menopause, men younger than 50 years, and children. In such cases, the diagnosis is based on ≤−2.0, which means BMD below the ‘normal’ BMD expected for age and gender, suggesting the need to investigate pathological causes. | • Calcium intake: women aged>50 years=1200mg/day (supplements: 600mg of elemental calcium+200IU or 400IU of cholecalciferol)• Vitamin D: adults aged>50 years=800–1000IU/day of vitamin D3 (cod liver oil, fortified milk, egg yolk, fresh salmon, canned tuna, canned sardine)• Exercises that increase muscle strength against gravity (bodybuilding, local exercises, etc.)• Prevention of falls• Smoking cessation• Avoid excessive alcohol consumption.Avoid the use of steroids and proton-pump inhibitors.Pharmacological treatment:- Antiresorptive agents: bisphosphonates (alendronate, risedronate, ibandronate, zoledronic acid); selective estrogen receptor modulators (raloxifene); hormonal therapy- Bone forming or anabolic: teriparatide- Bone forming/antiresorptive: strontium ranelateThe use of calcium and bisphosphonates is recommended in cases of fractures or BMD T score<2.5 standard deviations. |
| Prostate cancer | • Familial history• Afro-descendants• Fat-rich diet | • Prostate assessment (digital rectal exam – DRE)• Assessment of the blood prostate-specific agent (PSA) levels• Transrectal ultrasound• Diet low in fat and high in protein, fruits, vegetables, and legumes• The American Urological Association recommends PSA and DRE in asymptomatic men aged 40 years or older when the life expectancy is greater than 10 years. This recommendation is currently being updated.• The American Cancer Society recommends that men at average risk be given information starting at age 50 and black men and those with familial history of prostate cancer at age 45.• The American College of Preventive Medicine recommends that general practitioners discuss the potential benefits and risks of PSA screening with men aged 50 years or older while taking the patients’ preferences into account and making individual screening decisions.• Guidelines for HIV-infected patients are being established. Earlier prostate cancer screening based on an HIV diagnosis does not show patent advantages. |
| Renal function | • Age• Diabetes• CVD• Deficient nutrition• HIV: directly or indirectly causes several types of kidney disorders such as nephropathy, thrombotic microangiopathy, immune-mediated glomerulonephritis• Some ART agents might cause or exacerbate pre-existing nephropathy. | • Verify the glomerular filtration rate (GFR):Grade 1: Kidney alterations with normal or increased GFR; GFR>90mL/min/1.73m2Grade 2: Kidney alterations with slightly decreased GFR; GFR=30–59mL/min/1.73m2Grade 3: Moderate reduction of GFR; GFR=30–59mL/min/1.73m2Grade 4: Severe reduction of GFR; GFR=15–29mL/min/1.73m2Grade 5: Kidney failure; GFR<15mL/min/1.73m2 (or under dialysis)• Avoid combinations of nephrotoxic drugs.• Smoking and alcoholism cessation and a healthy diet• Treat dyslipidemia and diabetes.• Adjust dose of medications when needed. |
| Skin cancer | • Light skin | • Use of sun protection• Avoidance of excessive sun exposure |
| Uterine cancer | • Social factors (low socioeconomic class)• Habits (poor hygiene; prolonged use of oral contraceptives)• Sexual activity and pregnancy before age 18• Smoking (directly related with the number of cigarettes)Infection by HPV and herpes virus type 2 (HSV-2)• Multiple sexual partners | • Annual preventive cervical cancer exam (Papanicolaou, test)• For cervical cancer screening, the Pap smear must be performed during anogenital examination after the diagnosis of HIV, with a second Pap smear six months later and then once a year when the results are normal.• Colposcopy in indicated cases: whenever Pap is abnormal• HPV testing as a cervical cancer screening method in HIV-infected women might also be efficacious. |
| Vaccination | Vaccination decisions depend on the clinical and vaccination history of patients.The response to vaccines is better in patients with CD4+>350cells/mm3. Assess the best time to vaccinate the patient, but do not delay the onset of vaccination in high-risk patients.Inactivated vaccines have no restrictions in immunodeficient individuals. Attenuated vaccines must only be used when the potential benefit outweighs the risk and only in patients with CD4+>200cells/mm3.Some vaccines might transiently increase the viral load without clinical consequences. It is recommended to vaccinate during the intervals between viral load tests. | • Inactivated DT: Basic schedule: 3 doses (at 0, 2, and 4 months; booster: 1 dose every 10 years)• Influenza: 1 dose every year• Pneumococcus (Streptococcus pneumoniae) – inactivated pneumo 23-valent: 1 intramuscular or subcutaneous dose with a single booster 5 years later; vaccination reduces the risk of pneumonia and invasive disease• Attenuated varicella: susceptible adults with CD4+>200cells/mm3: 2 doses with a 4–8-week interval• HPV: tetravalent (HPV 16, 18, 6, 11) – ideally, vaccinate both males and females between the ages of 9 and 26 (3 doses: 0, 2, and 6 months). Not available in the Brazilian public health network, and its cost is high. Use is safe in HIV-infected individuals; however, cost-effectiveness studies must be performed in this population.• Attenuated yellow fever: upon traveling to risk area. When CD4+>200cells/mm3: 1 dose every 10 years |
Health care and prevention guidelines tailored to elderly HIV+ individuals are needed. The higher prevalence of comorbidities, polypharmacy, drug interactions, and end-organ disease in this patient population requires a multidisciplinary approach. Longitudinal studies are needed to assess the actual impact of HIV on aging and vice versa. In addition, HIV prevention strategies targeted to older adults should be considered.
Conflict of interestAll authors declare to have no conflict of interest.