Are we prepared to face newly emerging viral diseases? This question is as recurrent as the emergence of new viruses and the reemergence of neglected infectious diseases. Of note, Central and South America are considered world hotspots for the emergence of new mammalian viral zoonoses.1 Due to the size of its territory, Brazil lies at the center of these hotspots.
An assessment of viral diversity across mammalian orders has identified bats as the main reservoir of new viruses in Brazil.1 As one of the world's most biodiverse countries, Brazil harbors a large diversity of bat species, as well as a number of species that might act as hosts of as yet unknown pathogens. In this context, the arrival of exogenous viruses and their adaptation to our diverse environmental and socio-ecological conditions should also be taken into consideration.
The yellow fever outbreak this year and the recent spread of Zika virus (ZIKV) from Brazil to other American countries2 are poignant examples of the failure of our country's strategies for monitoring and controlling outbreaks and epidemics. They clearly reflect a lack of political interest in basic public health measures and epidemiological surveillance. At the same time, they result from failure to implement new technologies for population-level monitoring of virus circulation between humans and zoonotic animals.
To avoid potentially serious mistakes, such as the late detection of ZIKV in our country, a different attitude is required. In addition to improve vector control of known diseases, such as arthropod-borne infections, surveillance of the circulation of viruses among bats and other mammalian species in the Brazilian territory must be reinforced. In addition, of obvious interest to public health, the rapid detection of new viruses among humans is essential.
We believe that a rapid detection of zoonotic spillovers will only be possible if our knowledge about the viruses circulating in non-human animals of high zoonotic potential is channeled into a powerful and effective surveillance system. According to Plowright et al.,3 a “zoonotic spillover” is defined as the “transmission of a pathogen from a vertebrate animal to a human”. We would like to highlight the importance of detecting when a pathogen crosses the boundary from its natural reservoir to start circulating among humans, a complex event involving environmental, pathogen, and host factors.3 Following a zoonotic spillover, human-to-human transmission is essential to sustain an epidemic or pandemic.3,4 As a matter of fact, most zoonotic viruses will never cause infections that spread extensively among the human population.4 Nevertheless, viruses hitherto attracting little medical interest can unexpectedly become a threat to public health. For example, until the recent discovery of the link between ZIKV and microcephaly and other congenital problems, ZIKV infection was not considered clinically or epidemiologically important.
Efforts toward the surveillance of virus circulation in humans can build on samples collected for other purposes.2 For example, the screening of blood bank samples for the presence of zoonotic viruses might represent a promising strategy requiring a relatively minor investment. As previously discussed,5 we encourage the use of broad spectrum methods for the detection of emerging epidemics and pandemics. Monitoring approaches based on the simultaneous detection of genomic sequences of different viruses appear to be both cost-effective and fast, while covering a broad spectrum of pathogens.2,5 However, we understand the importance of focusing monitoring efforts on those human groups most susceptible to coming into contact with new viruses. Moreover, considering the vast number of animal pathogens,3 monitoring strategies should target (I) viruses showing a particularly high potential for human-to-human transmission, and (II) viruses already circulating in other human populations. Indiscriminate surveys may not be effective in predicting pandemics.4
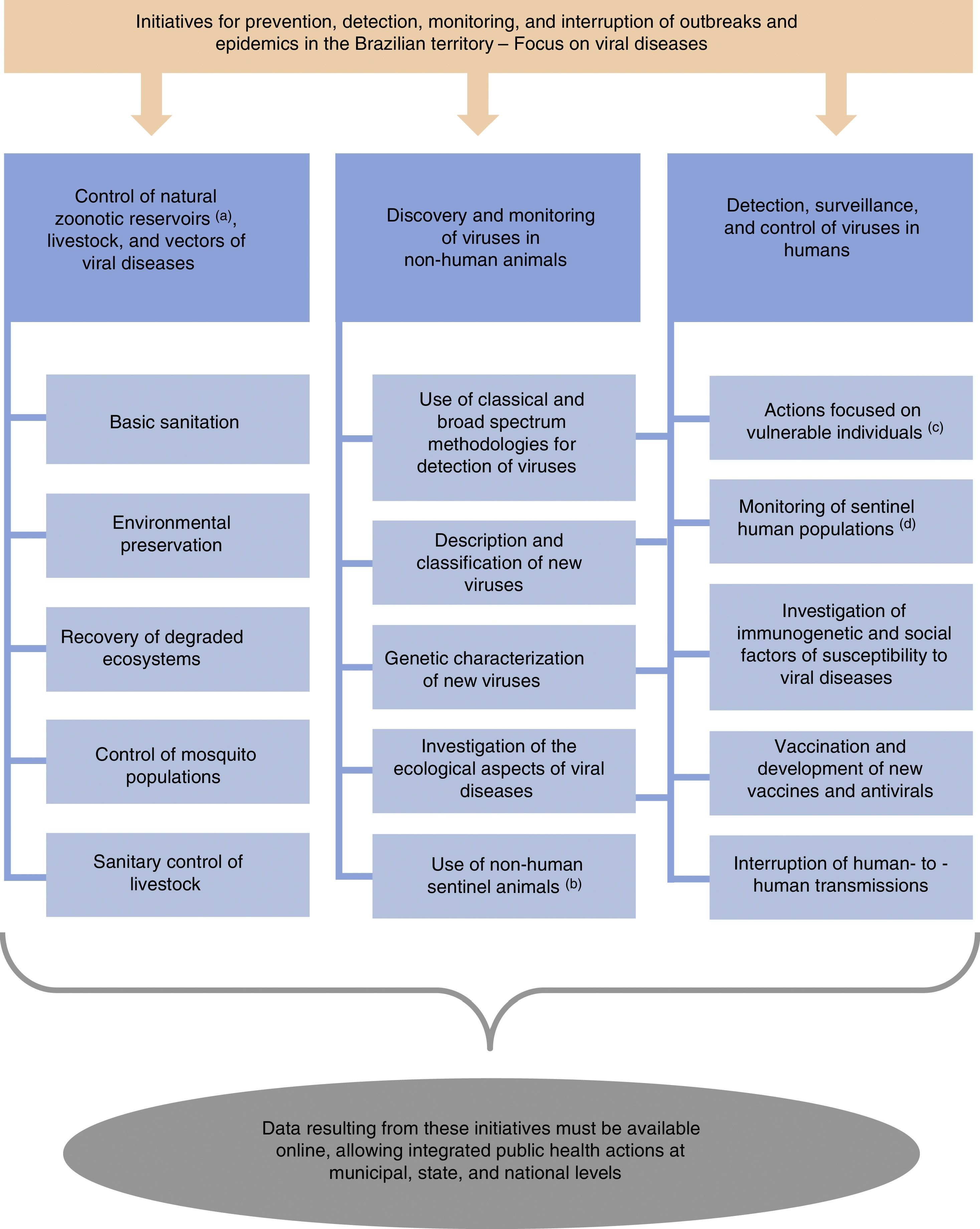
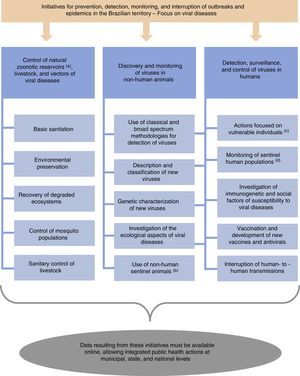
Here, we suggest a number of initiatives for the prevention, detection, monitoring, and interruption of outbreaks and epidemics in Brazil (Fig. 1). Additional actions may be suggested and applied to complement these initiatives. Importantly, our initial suggestions must be viewed in the context of a best case scenario in terms of political, economic, and public health conditions in Brazil. Under current circumstances, the implementation of various of these initiatives will be difficult or remain incomplete. Despite the potential difficulties, the measures suggested here are important to improve the Brazilian system for epidemiological control of viral pathogens. The resources needed to stop or mitigate an epidemic would certainly greatly exceed those necessary to finance prevention strategies. Finally, these measures suggested by us are essential to prevent Brazil from becoming, yet again, a nursery for new zoonoses.
Initiatives for prevention, detection, and interruption of outbreaks and epidemics in the Brazilian territory, with focus on viral diseases. aBats, dogs, cats, rodents, pigeons, among others. bNon-human animals experimentally or naturally exposed to risk areas. cMainly injecting drug users and sex workers (these populations are more susceptible to viral transmission among humans). dHunters, individuals living in rural areas, and those in close contact with livestock (these groups of people are more susceptible to zoonotic spillover). In addition, blood donors or individuals who consent to donate biological samples for research activities can be used as human sentinels in strategies for the surveillance of zoonotic diseases.
The authors declare no conflicts of interest.