Erythema multiforme (EM), Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis (TEN) have been reported as possible adverse effects of some classes of first-line antiretroviral drugs (ART) for HIV treatment. Herein we report an unusual presentation of TEN lesions associated with ART in an HIV-infected patient. The patient presented disseminated cutaneous eruption and oral lesions from the lips to the oropharynx region, causing odynophagia and dysphagia. In the tongue, circular, atypical erythematous lesions appeared, increasing in diameter over seven days and coalescing since then to complete remission. TEN treatment included efavirenz interruption, use of methylprednisolone, prophylactic antibiotic, and daily laser therapy with low-intensity red light. The circular oral lesions have not been described yet. Reporting our findings and clinical management may help diagnosing other similar cases and guide the clinical conduct. Analgesia and acceleration of oral ulcer repair with red laser therapy are recommended.
The aim of antiretroviral therapy (ART) is to suppress plasma HIV replication, restore and preserve immunologic function reducing HIV-associated morbidity, extending the duration and quality of survival, and preventing HIV transmission.1 Most current recommendations indicate early ART initiation regardless of CD4+ T lymphocyte cell count.1–3
The most used first-line ART regimens are combinations of three different antiretroviral drugs, including two nucleoside reverse transcriptase inhibitors plus one of the following: integrase inhibitor, non-nucleoside reverse transcriptase inhibitor, or protease inhibitor with a pharmacokinetic enhancer.1,2
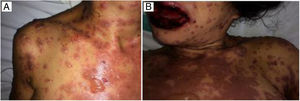
The Brazilian Ministry of Health recommends two nucleoside reverse transcriptase inhibitors plus an integrase inhibitor.3 Some non-nucleoside reverse transcriptase inhibitors, such as efavirenz (EFV) or nevirapine (NVP),4–8 or even nucleoside reverse transcriptase inhibitors, such as emtricitabine and lamivudine,9 have been associated with immune-mediated dermatological reactions such as erythema multiforme (EM), Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). These reactions are characterized by extensive areas of skin scaling, affect multiple organs and systems, and could potentially have fatal outcomes.
SJS, overlap SJS/TEN and TEN are usually drug-induced pathologies.10 The usual distribution of body surface area detachment in EM is below 10%, presenting localized typical or atypical targets, while SJS involves the body surface area detachment below 10% with widespread erythematous, purpuric macules or flat atypical targets. In overlap SJS/TEN, the body surface area detachment is between 10% and 30%, presenting widespread purpuric macules or flat atypical targets.10 EM skin lesions are commonly described as “target”.11–13 SJS/TEN may present mucous membranes involvement, with erythematous, bleeding, painful erosions on the oral mucosa and lips, which can also be found on ocular and anogenital mucosa.14,15 Treatment of these conditions, although controversial, usually involves the disease-triggering drug interruption and management of the bare skin areas, infection prevention and minimization of pain.15,16 Oral lesions usually disappear slowly, but the use of laser therapy can accelerate tissue recovery and provide fast analgesia.17
This report describes a clinical case of TEN in an HIV-infected patient who developed TEN, with unusual severe oral lesions after efavirenz use, and a treatment based on low intensity red laser photobiomodulation. The reported case was approved by the Ethical Review Board, Edgard Santos University Hospital (protocol number 1740483). The patient signed an informed consent form.
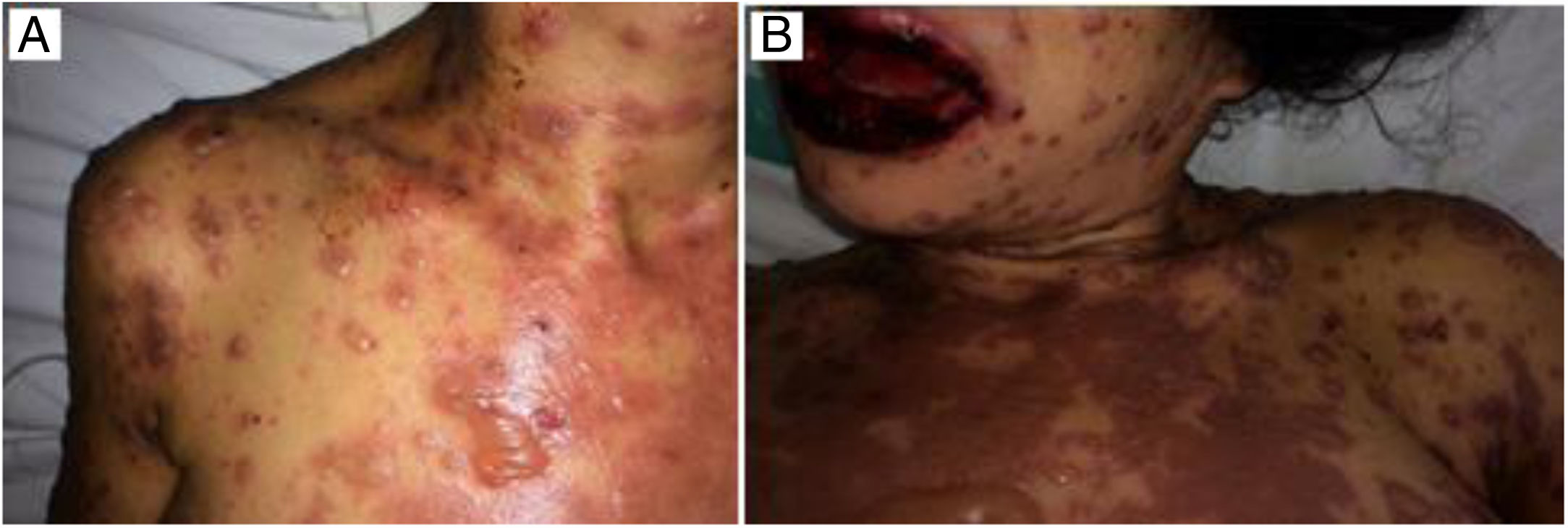
Case reportMCN, a 35-year-old female, diagnosed with AIDS in 2012, was on Tenofovir, Lamivudine, Atazanavir/ritonavir since diagnosis. In June 2016, the patient was hospitalized due to pericardial effusion secondary to pulmonary tuberculosis, which was treated with surgical drainage and standard tuberculosis therapy (rifampicin, isoniazid, pyrazinamide, and ethambutol). At that time, the patient had undetectable HIV RNA level and CD4 count of 177cells/ml. In January 2017, after treatment failure, ART was changed to Tenofovir, Lamivudine and Efavirenz. Eight days after ART switch, she presented fever, erythematous spots, papules and pruritic bullous lesions all over the body (Fig. 1). The patient developed ocular hypersecretion, conjunctivitis, reduced vision, odynophagia and swallowing pain. She was then admitted to Federal University of Bahia Hospital, Salvador, Bahia, Brazil.
At hospital admission, the patient presented regular clinical conditions, without fever or respiratory distress. She was still on rifampicin 150mg, isoniazid 75mg, pyrazinamide 400mg, and ethambutol 275mg. The suspected diagnosis was TEN secondary to Efavirenz use. ART and anti-tuberculosis treatment were discontinued and intravenous (IV) therapy with Methylprednisolone 40mg/day was administered. On the following days, she developed tachycardia, fever, difficulty in opening the eyes and dysuria. The patient was placed on isolation precautions to prevent secondary infection. Prophylaxis with sulfamethoxazole 800mg/trimethoprim 160mg was started. Methylprednisolone 40mg/day was tapered off to 10mg/day within four weeks when it was replaced by oral prednisone 5mg daily. For the ocular lesions, the patient used ofloxacin 3mg/ml and dexamethasone 1mg/ml eye drops and irrigation with 0.9% saline solution. The patient presented pyuria, associated with suprapubic pain and was treated with Imipenem 500mg/Cilastatin 500mg for seven days due to the isolation of an ESBL-producer E. coli in urine culture.
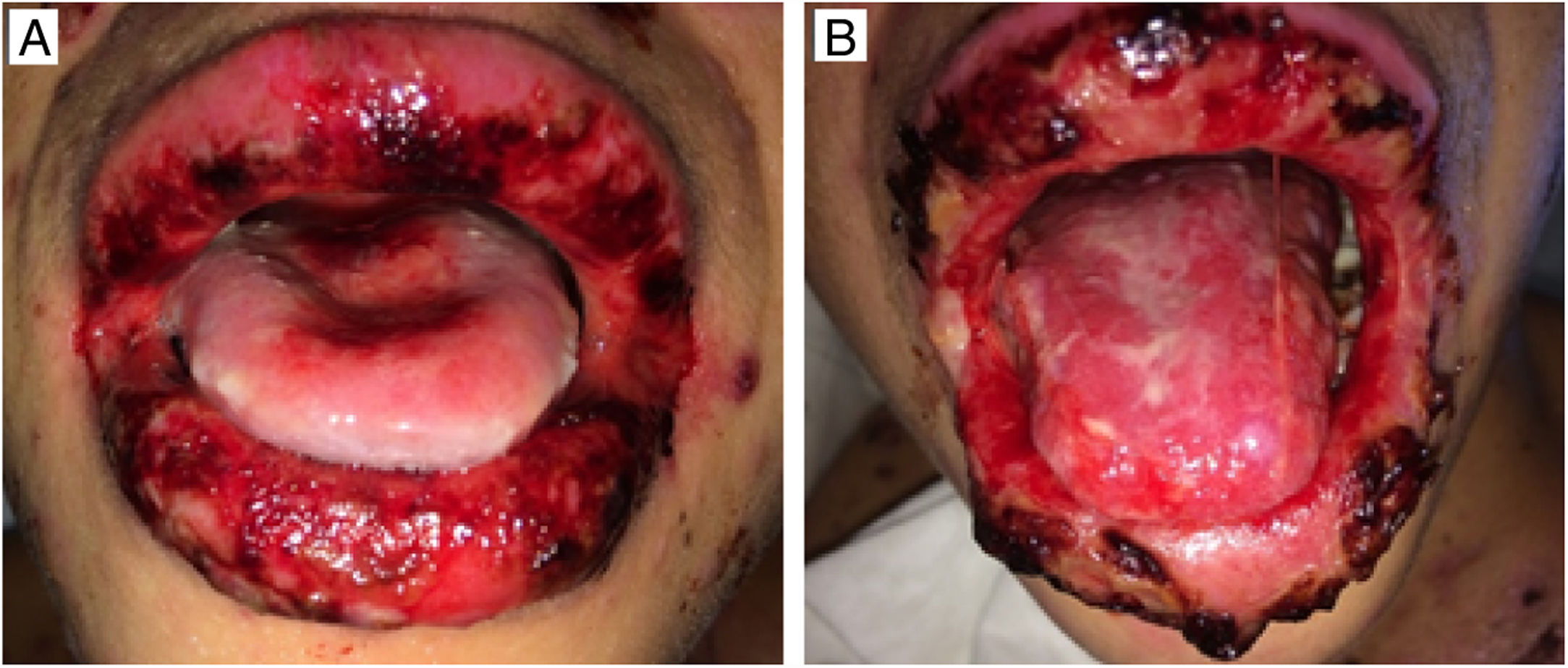
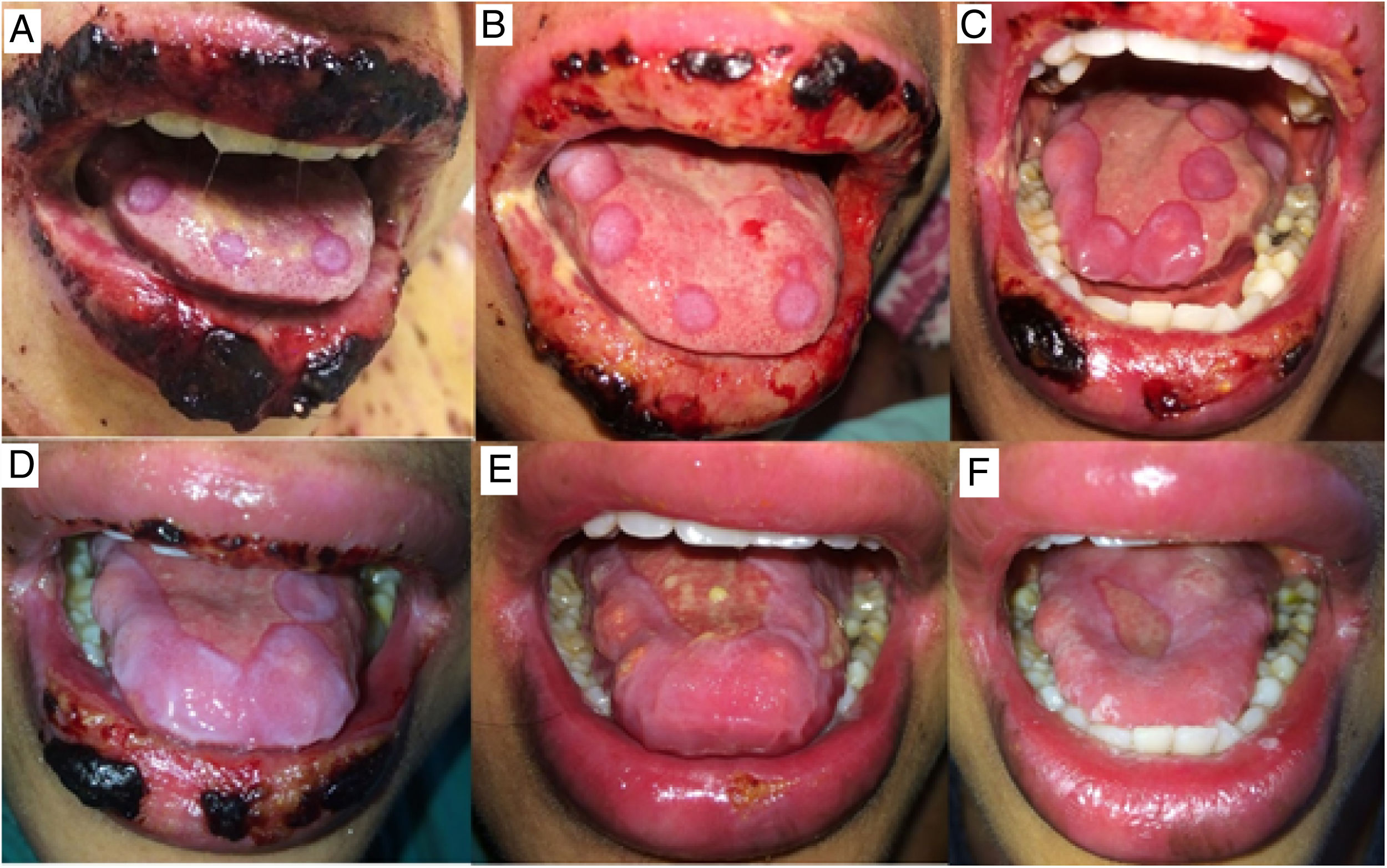
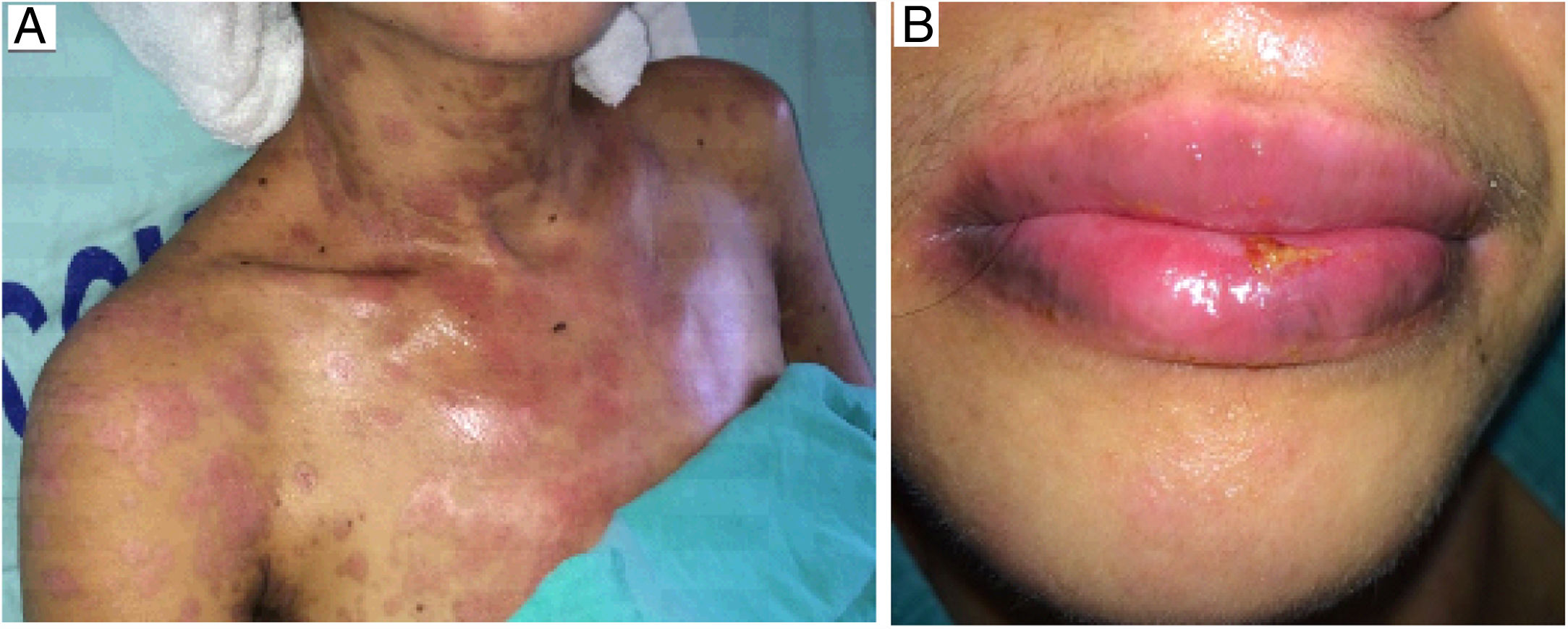
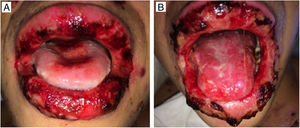
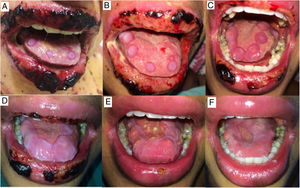
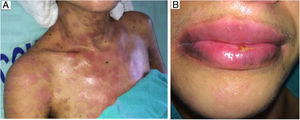
The oral lesions spread from the lips to the oropharynx region within two days, causing pain and incapacity to ingest solids. On the lips, hemorrhagic lesions formed crusts (Fig. 2). Some circular, atypical erythematous lesions appeared in the tongue, increasing in diameter over the following seven days and coalescing at the end (Fig. 3). Daily low-intensity red laser (5J/cm2) therapy for two weeks was applied in addition to mouthwash with non-alcoholic chlorhexidine (0.12%) twice a day and lips hydration until remission of oral lesions. The patient was discharged after 30 days of hospital admission, with complete healing of cutaneous and mucosal lesions (Fig. 4). ART (atazanavir, ritonavir, lamivudine, tenofovir) was resumed, and the patient was kept on sulfamethoxazole (800mg)/trimethoprim (160mg) and oral prednisone (5mg).
A retrospective review of patients hospitalized in the dermatology ward from 1992 to 2012 showed that SJS and TEN were the major cause of hospitalization. Sixty-three percent of these patients were HIV-infected, evidencing the susceptibility of this population to adverse reactions.18 Inappropriate or unnecessary use of drugs like trimethoprim-sulfamethoxazole, atovaquone, clindamycin, and fluconazole, in HIV-infected patients may cause severe adverse drug reactions.19
Some studies have shown that SJS and TEN may also result from exposure to drugs used in ART regimens, such as efavirenz and nevirapine.4,5 Reddy et al.4 reported nine cases of SJS and one of TEN associated with the use of nevirapine in patients living with HIV/Aids. A case report has also associated higher doses of nevirapine with SJS in a patient who was using no other medication, had undetectable viral load and a CD4 count higher than 350 cells/ml. The patient tried to compensate a missing dose of his ART regimen (nevirapine, tenofovir and lamivudine) by doubling the regular dose for two days.5 Nevirapine toxicity has been associated with high CD4 counts, undetectable viral load and high plasma drug level. Authors postulate that the sudden increase of plasma levels of nevirapine, in a patient on those conditions, has triggered SJS. In 2016, a case of fatal nevirapine-induced TEN in an HIV-infected patient has been reported.6 This association was made based on temporal relationship between drug use the hypersensitivity reaction, and associated clinical signs and symptoms.
In a case-control study,7 nevirapine exposure was strongly associated with SJS or TEN in HIV-infected patients, but no association was found with efavirenz, which has been associated with a lower frequency of severe hypersensitivity events when compared to nevirapine.8
Another report describes a clinical picture similar to the present case, in which the development of dermatological lesions occurred two weeks after the introduction of efavirenz (600mg once daily).15 In the present case, the symptoms started one week after efavirenz introduction. The causality assessment showed strong clinical correlation between efavirenz use and the onset of dermatological lesions. In both cases, anti-tuberculosis drugs and other ART drugs were already in use and well tolerated. This fact reinforces that those drugs were likely not implicated in disease causality.
Early recognition of the drug reaction and discontinuation of the presumptive drug are very important in SJS management, as well as supportive care provision.15 Corticosteroids are effective in the initial phase, but they may result in increased risk of infection and delayed wound healing, particularly during the bullous eruption or mucosal erosion.15 Administration of short-term dexamethasone pulse therapy, particularly during the initial phase of TEN, may be beneficial in reducing the mortality rate.16 However, it has been reported that neither intravenous immunoglobulins nor corticosteroids showed any significant effect on mortality in comparison with supportive care alone.20
Effective treatment with intravenous fluid, corticosteroids, and oral antihistamines has also been reported.15 Treatment of skin lesions with topical application of mupirocin 0.9% NaCl and 0.5% AgNO3 three times a day for 14 days has been described. Ocular and mucosal lesions are usually managed with supportive therapy. Successful use of laser phototherapy (LPT) for treating oral lesions has also been reported.17
In the present case, we administered 40mg of IV methylprednisolone, associated with antimicrobial therapy, to avoid secondary infection of lesions. Dexamethasone eye drops was also used. IV corticosteroids was replaced by oral prednisone; at hospital discharge, the patient was on low maintenance dose (5mg/day) of prednisone. This therapeutic approach, in association with efavirenz interruption and low-intensity red laser therapy, were effective in the present case, with gradual remission of the oral lesions, without secondary infection.
This case draws the attention to the presentation of oral lesions, not yet reported in the literature, which may help diagnosing other similar cases. This also indicates the need for a greater awareness on skin reaction in patients starting efavirenz, as well as the need for strict adherence to medical recommendations when efavirenz is introduced. Analgesia and acceleration of oral ulcer repair with red laser therapy are recommended in patients in these circumstances, to minimize pain and discomfort and to allow adequate patient feeding.
Conflicts of interestThe authors declare no conflicts of interest.
FundingThis work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.