Brucellosis is an infectious, contagious and zoonotic disease that occurs worldwide. The family members of an index case of brucellosis may be especially susceptible, due to sharing the same source of infection and similar risk factors for brucellosis. In this study, we propose to screen pediatric and adult family members of brucellosis index cases for detecting additional unrecognized infected family members.
Materials and methods114 family members of 41 pediatric patients with brucellosis were evaluated. All family members completed a brief questionnaire and were tested by a standard tube agglutination test (STA).
ResultsThe majority of family members (n=96, 84.2%) were children. Among the 114 family members, 42 (36.8%) were seropositive, and 15 (35.7%) were symptomatic. The majority of the symptomatic seropositive family members (n=12, 80%) had STA titers (≥1:640) higher than asymptomatic seropositive family members (n=9, 33%; p=0.004).
ConclusionThe routine screening of both pediatric and adult family members of index cases is a priority in endemic areas. Using this screening approach, unrecognized family members who are seropositive for brucellosis will be identified earlier and be able to receive prompt treatment.
Brucellosis is thought to be the most common zoonosis worldwide1; while it is an important public health issue throughout the world, it is of especial concern in the Mediterranean region, including Turkey.1–3 The incidence of this zoonotic disease in Turkey is 23 per 100,000 yearly.3 Brucellosis is especially common in the rural areas of the middle and south-eastern Anatolian regions. Brucella melitensis is the most prevalent strain.4
Brucellosis is a contagious disease of animals that is transmissible to humans, who are accidental hosts. It is transmitted to humans by contact with infected animals, their carcasses, excretions, or through derived food products like unpasteurized milk and cheese.5,6 Therefore, individuals recognized to be at increased risk include farmers, veterinarians, abattoir workers, and laboratory personnel. Transmission from human to human is rare, as are transplacental, breast-feeding, and venereal transmissions.5 It is a systemic infection which may involve any organ or system of the body.
In endemic areas with a potentially high rate of population exposure, brucellosis should be considered one of the differential diagnoses for any patient suffering from indefinite involvement of miscellaneous organs, because early diagnosis and early treatment of infected cases can reduce the rate of complications.7 Unrecognized seropositive individuals may include family members of the index case, who share the same source of brucellosis and similar genetic and environmental risk factors for the disease.8,7,9
In this study, we screened pediatric and adult family members of index cases with brucellosis to detect unrecognized infected family members. In addition, we evaluated the demographic, epidemiologic, clinical, and serological features of family members and compared seropositive and seronegative family members.
Materials and methodsA total of 114 family members of 41 pediatric patients (index cases) who had been diagnosed with acute brucellosis at the Gaziantep State Children Hospital's Department of Pediatric Infectious Diseases were evaluated during the period from September 2012 to August 2013. The diagnosis of acute brucellosis was based on the US Centers for Disease Control definition, which includes the presence of clinical signs and symptoms with evidence of Brucella spp. invasion in positive culture or a single standard tube agglutination test (STA) against Brucella spp. of ≥1:160.10 Pediatric and adult family members screening was initiated after diagnosis of the index case; all family members completed a brief questionnaire. The questions included demographic data of the index case and family members, such as age, sex, and epidemiological family data like living area, animal ownership, handling of animals or their excretions, and previous history of brucellosis. Any history of drinking unpasteurized milk and consumption of unpasteurized dairy products such as cream and cheese were also recorded.
Family members of the pediatric index case were evaluated by a pediatric infectious diseases specialist and adult family members were evaluated by an infectious diseases specialist. Family members of index cases were tested by slide agglutination tests (Rose Bengal test). Family members who had positive results were further tested by STA. A seropositive family member was defined as an individual with STA titer≥1:160, while a seronegative family member was defined as an individual with STA titer≤1:80. Blood samples were not cultured for Brucella sp. routinely, although some were cultured. Physical signs and symptoms of index cases and symptomatic seropositive family members were recorded.
Data analysis was performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as median and mean±S.D. (standard deviation); Chi-square test was used to compare proportions. Fisher's exact test was used for expected frequencies below five in contingency tables. p-Values <0.05 were considered statistically significant.
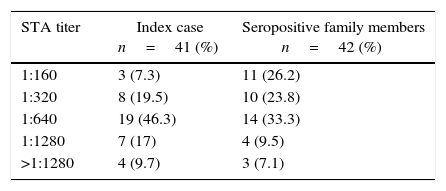
ResultsForty-one pediatric index cases and 114 family members were included in this study. The age distribution of the index cases was 1–18 years, with a median age of 8.1 years; 26 (63.4%) were boys and 15 (36.6%) girls. The most frequent symptoms were fever, malaise and arthralgia. The physical signs and symptoms of index cases are reported in Table 1. Their STA titers ranged from 1:160 to >1:1280; the titers were 1:160 in three (7.3%) cases, 1:320 in eight (19.5%), 1:640 in 19 (46.3%), 1:1280 in seven (17%), and ≥1280 in the remaining four (9.7%) index cases (Table 2).
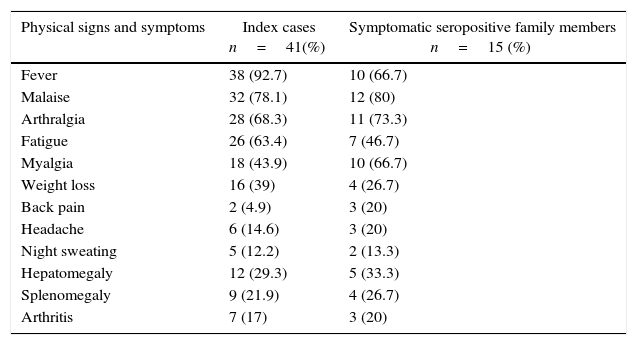
Physical signs and symptoms of Brucellosis index cases and symptomatic seropositive family members.
| Physical signs and symptoms | Index cases n=41(%) | Symptomatic seropositive family members n=15 (%) |
|---|---|---|
| Fever | 38 (92.7) | 10 (66.7) |
| Malaise | 32 (78.1) | 12 (80) |
| Arthralgia | 28 (68.3) | 11 (73.3) |
| Fatigue | 26 (63.4) | 7 (46.7) |
| Myalgia | 18 (43.9) | 10 (66.7) |
| Weight loss | 16 (39) | 4 (26.7) |
| Back pain | 2 (4.9) | 3 (20) |
| Headache | 6 (14.6) | 3 (20) |
| Night sweating | 5 (12.2) | 2 (13.3) |
| Hepatomegaly | 12 (29.3) | 5 (33.3) |
| Splenomegaly | 9 (21.9) | 4 (26.7) |
| Arthritis | 7 (17) | 3 (20) |
The distribution of standard tube agglutination test (STA) titers of Brucellosis index cases and seropositive family members.
| STA titer | Index case n=41 (%) | Seropositive family members n=42 (%) |
|---|---|---|
| 1:160 | 3 (7.3) | 11 (26.2) |
| 1:320 | 8 (19.5) | 10 (23.8) |
| 1:640 | 19 (46.3) | 14 (33.3) |
| 1:1280 | 7 (17) | 4 (9.5) |
| >1:1280 | 4 (9.7) | 3 (7.1) |
STA, standard tube agglutination test.
Out of the 114 family members, 96 (84.2%) were children and 18 (15.8%) were adults, age range 2–54 years, median 13.4 years; there were 60 (52.6%) males and 54 (47.4%) females (p=0.66). Seventy two family members (63.2%) lived in rural areas and 42 in urban areas (36.8%) (p=0.01). Thirty family members (26.3%) owned animals: 23 (20.2%) had sheeps, two (1.7%) had cows, and five (4.4%) had cows and sheeps. Eight family members (7%) had a history of handling animals or animal excretions. None of them admitted drinking unpasteurized milk, but 75 (65.7%) family members had a history of ingesting unpasteurized dairy products: 61 (53.5%) eating fresh cheese, seven (6.1%) using cream, and seven (6.1%) eating both fresh cheese and cream. Previous history of brucellosis was reported by two (1.75%) family members, one of whom was seropositive and the other seronegative.
Among the 114 family members, 42 (36.8%) were seropositive with a brucella antibody titer ≥1:160 and 72 (63.2%) were seronegative with a brucella antibody titer ≤1:80. Among the seropositive individuals, 27 (64.3%) were asymptomatic and 15 (35.7%) were symptomatic, of whom 10 (66.7%) were children. Among symptomatic family members, the most frequent symptoms were fever, malaise, and arthralgia (Table 1). Eleven (15.2%) of the 72 seronegative family members were symptomatic.
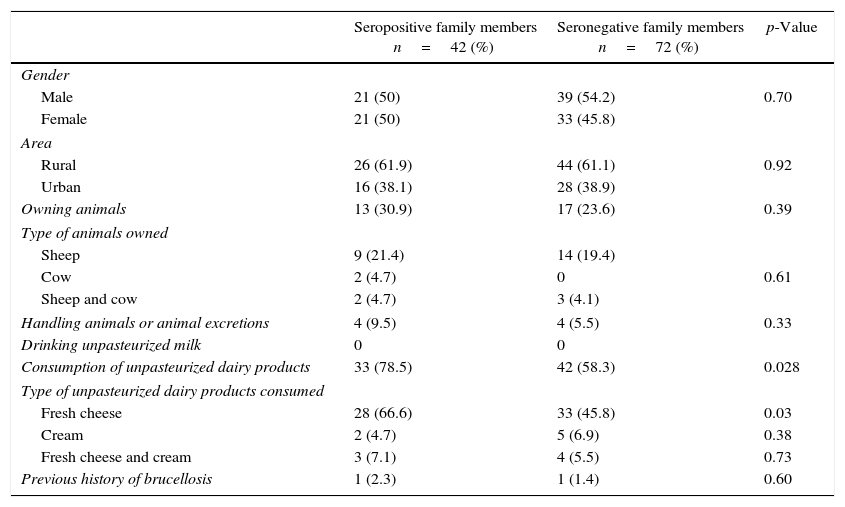
Some of the epidemiological and demographical characteristics of both seropositive and seronegative family members are shown in Table 3. There were no major epidemiological and demographical differences between seropositive and seronegative family members, but consumption of unpasteurized dairy products, especially fresh cheese, was higher among seropositive family members than in seronegative family members (p=0.028, p=0.03; respectively).
Epidemiological and demographical characteristics of both seropositive and seronegative family members of children with acute brucellosis.
| Seropositive family members n=42 (%) | Seronegative family members n=72 (%) | p-Value | |
|---|---|---|---|
| Gender | |||
| Male | 21 (50) | 39 (54.2) | 0.70 |
| Female | 21 (50) | 33 (45.8) | |
| Area | |||
| Rural | 26 (61.9) | 44 (61.1) | 0.92 |
| Urban | 16 (38.1) | 28 (38.9) | |
| Owning animals | 13 (30.9) | 17 (23.6) | 0.39 |
| Type of animals owned | |||
| Sheep | 9 (21.4) | 14 (19.4) | |
| Cow | 2 (4.7) | 0 | 0.61 |
| Sheep and cow | 2 (4.7) | 3 (4.1) | |
| Handling animals or animal excretions | 4 (9.5) | 4 (5.5) | 0.33 |
| Drinking unpasteurized milk | 0 | 0 | |
| Consumption of unpasteurized dairy products | 33 (78.5) | 42 (58.3) | 0.028 |
| Type of unpasteurized dairy products consumed | |||
| Fresh cheese | 28 (66.6) | 33 (45.8) | 0.03 |
| Cream | 2 (4.7) | 5 (6.9) | 0.38 |
| Fresh cheese and cream | 3 (7.1) | 4 (5.5) | 0.73 |
| Previous history of brucellosis | 1 (2.3) | 1 (1.4) | 0.60 |
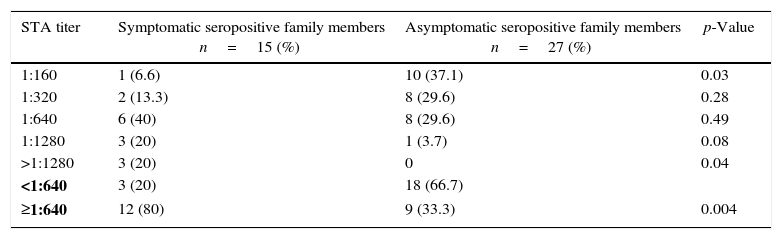
In seropositive family members, STA titers ranged from 1:160 to >1:1280, namely 1:160 in 11 (26.2%), 1:320 in 10 (23.8%), 1:640 in 14 (33.3%), 1:1280 in 4 (9.5%), and ≥1280 in 3 (7.1%) (Table 2). STA titers were compared between symptomatic and asymptomatic seropositive family members; the results are shown in Table 4. In asymptomatic seropositive family members, 10 (37.1%) had an STA titer of 1:160 whereas only one (6.6%) symptomatic seropositive had an STA titer (p=0.03). Three of the symptomatic seropositive family members (20%) had an STA titer of ≥1280, while no asymptomatic seropositive had a titer that high (p=0.04). Other STA titers of seropositives were not significantly different between symptomatic and asymptomatic family members (p>0.05 overall). The majority of symptomatic seropositive family members (n=12, 80%) had a higher STA titer (≥1:640) than asymptomatic seropositives (n=9, 33%). These differences were statistically significant (p=0.004).
The distribution of standard tube agglutination (STA) test titers of symptomatic and asymptomatic seropositive family members for Brucellosis.
| STA titer | Symptomatic seropositive family members n=15 (%) | Asymptomatic seropositive family members n=27 (%) | p-Value |
|---|---|---|---|
| 1:160 | 1 (6.6) | 10 (37.1) | 0.03 |
| 1:320 | 2 (13.3) | 8 (29.6) | 0.28 |
| 1:640 | 6 (40) | 8 (29.6) | 0.49 |
| 1:1280 | 3 (20) | 1 (3.7) | 0.08 |
| >1:1280 | 3 (20) | 0 | 0.04 |
| <1:640 | 3 (20) | 18 (66.7) | |
| ≥1:640 | 12 (80) | 9 (33.3) | 0.004 |
STA, standard tube agglutination test.
Symptomatic seropositive family members were treated, whereas asymptomatic seropositive family members were followed-up with no treatment unless they showed symptoms and an increase in STA titers. Of eight family members followed, two were diagnosed with acute brucellosis.
DiscussionTurkey has seven geographical regions and is an important migration route between Europe and Asia. There are significant differences between regions, in terms of geographical features and economic and social development. Due to the geographic situation, Turkey is a risk factor for many infectious diseases, such as brucellosis.11 A total of 189,226 cases of human brucellosis were officially reported between 2000 and 2005, of which approximately 90,000 were registered (approximately 15,000 cases per year) in Turkey. The highest seroprevalence was observed in the eastern and southeastern regions of the country.12 Despite being endemic in Turkey, brucellosis remains underdiagnosed due to its non-specific clinical manifestations and laboratory parameters.
In our study, the seroprevalence rate of brucellosis in family members of pediatric index cases was 36.8%. Tabak et al. evaluated 110 family members of Turkish index cases, and 20 (18.8%) were seropositive8; our results showed a comparatively higher rate. This difference may be due to the present study being conducted in the southeastern region of Turkey, which is a highly endemic area for brucellosis.
In 1954, Spink noticed that family members of brucellosis index cases were at risk of acquiring the disease.13 However, several other studies have reported different seroprevalence rates. In Saudi Arabia, Almuneef et al. evaluated 404 family members of 55 index cases, and detected 53 (13.6%) seropositive individuals.7 Another study in Saudi Arabia reported a brucellosis seroprevalence rate in family members of 19%.14 In Azerbaijan, Ismayilova et al. reported an even lower rate of 9.5%.9 The seropositivity rate in family members of index cases was higher in our study compared to these previous studies.
Fifteen (35.7%) of the seropositive family members were symptomatic, of whom 10 (66.7%) were children. Among seronegative family members, 11 (15.2%) were symptomatic, the most frequent symptoms being fever, malaise, and arthralgia; the diagnosis of brucellosis can be very challenging due to these non-specific clinical manifestations. These family members would not have been diagnosed without proactive screening.
Tabak et al. reported eight symptomatic individuals out of 20 seropositive family members.8 In a study from Saudi Arabia, 39 (74%) of 53 seropositive family members were symptomatic.7 Gotuzzo et al. evaluated 232 family members in Peru, and reported a high rate (50.9%) of symptomatic infections. They also observed that treatment of brucellosis in the early stages of the disease resulted in better outcomes than deferred treatment.15 Symptomatic seropositive family members were more likely to have high STA titers compared to their asymptomatic counterparts.14–16 Similar to these results, we found that symptomatic seropositive family members had higher anti-Brucella titers than asymptomatic cases.
Some of the published studies did not evaluate the clinical status of family members.7,17 In our study, the demographic, epidemiologic, clinical, and serologic features of the family members were evaluated, and the results were compared between the seropositive and seronegative family members. The majority of brucellosis index cases and family members were adults in most studies on this topic. However, all of the index cases and the majority of family members in our study were children. We acknowledge that the lack of a control group (individuals without index cases) was a limitation in our study design.
Family members of brucellosis index cases are at risk for the disease as they share the same brucellosis sources.7,18 Genetic factors are also important as environmental factors in the susceptibility to brucellosis that contributes to a high infection rate among family members.19,20 In this study, the consumption of unpasteurized dairy products, especially fresh cheese, was higher in seropositive family members than in seronegatives.
In conclusion, our results show that the seroprevalence rate of brucellosis in family members of pediatric index cases was 36.8%. The frequent occurrence of unrecognized brucellosis in this setting underscores the importance of routine familial screening for this disease. We confirmed that screening both pediatric and adult family members of index cases is an important issue in endemic areas. With this screening approach, unrecognized infected cases in family members, especially children, will be identified earlier in the course of the disease, and this early diagnosis and treatment will reduce complications.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Dr. Efgan Gayyurhan for his support.