The yeast phase of 22 Histoplasma capsulatum clinical isolates from Mexico, Argentina, Colombia, and Guatemala and three reference strains, one from Panama and two from the United States of America (USA), were screened for thermosensitivity characteristics using different analyses. Growth curves at 0, 3, 6, 12, 24, and 30 h of incubation at 37 and 40 °C, the growth inhibition percentage at 40 °C, and the doubling time at 37 and 40 °C were determined for all yeasts studied. Most of the isolates examined exhibited thermotolerant phenotypes at 40 °C, whereas a thermosensitive phenotype at 40 °C was only detected in the Downs reference strain from the USA. Growth inhibition values lower than 33.8% supported the predominance of the thermotolerant phenotype at 40 °C. The doubling time means found for the different isolates were 5.14 h ± 1.47 h at 37 °C and 5.55 h ± 1.87 h at 40 °C. This is the first report to underscore the predominance of thermotolerant and delayed doubling time phenotypes in H. capsulatum clinical isolates from different regions of Latin America.
The saprobe multicellular mycelial morphotype (M-phase) of the dimorphic fungus Histoplasma capsulatum develops in special environments at 25–28 °C. Aerosolized infective propagules of the M-phase, mainly microconidia and small hyphal fragments, can produce a respiratory infection when inhaled by humans and other mammals. Depending on the immunocompetence of the host, inoculum size, virulence, and phylogenetic species of the H. capsulatum isolate, the infection can lead to severe illness.1,2
The unicellular yeast morphotype (Y-phase) of this pathogen is related to intracellular parasitism in susceptible hosts (mainly immunocompromised individuals). The dimorphic transition of H. capsulatum is of particular interest because it is necessary for the manifestation of fungal virulence.3 Under laboratory conditions, the M- to Y-transition can be reversibly induced by temperature switches from 25 to 28 °C (M-phase) to 35–37 °C (Y-phase).
Critical studies about H. capsulatum phenotypes have focused on its morphology,4,5 thermosensitivity,6 and biochemical properties.7–9 Gass & Kobayashi10 described an atypical H. capsulatum strain, named Downs, isolated from an elderly patient with disseminated histoplasmosis, which showed thermosensitive phenotype at 40 °C and avirulence in mice. Spitzer et al.11 reported that fungal isolates from patients with Aids-associated histoplasmosis from St. Louis, Missouri, United States of America (USA), shared the same thermosensitivity characteristics of the Downs strain. This association was confirmed by molecular methods, which revealed that St. Louis H. capsulatum isolates had a polymorphic mtDNA profile similar to that of the Downs strain. According to the earlier classification of H. capsulatum proposed by Vincent et al.,12 all St. Louis isolates were included in a single group named Class 1. Later, the fungal strains from Class 1 were reclassified as North American Class 1 (NAm 1) phylogenetic species by Kasuga et al.,13 and NAm 1 strains have now been renamed by Sepúlveda et al.14 as H. mississippiense sp. nov. Formerly, NAm 1 strains were considered to have low virulence, though recent records have documented another NAm 1 strain developing a virulent phenotype under experimental assays.2
Overall, most phenotypic statements about H. capsulatum have resulted from the use of a limited number of isolates and considering as reference the same overmanipulated strains: Downs (low virulence)10 and G-217B (high virulence)5 from the USA and G-186B (high virulence)15 from Panama. Given that H. capsulatum is distributed between the 54 °N16 and 38 °S17 latitudes, its distinct phenotypes may be used to discriminate between several isolates from different geographic distributions, which will contribute to the understanding of this pathogen diversity.
The aim of the present study was to phenotype clinical isolates of H. capsulatum from different regions of Latin America considering their Y-phase thermosensitivity at 40 °C, through the detection of their growth curve at 37 and 40 °C, growth inhibition percentage (GI%) at 40 °C, and doubling time (Dt) at 37 and 40 °C.
Material and methodsHistoplasma capsulatumTwenty-two clinical isolates from four Latin American countries were processed, 11 from Mexico, five from Argentina, five from Colombia, and one from Guatemala, together with three clinical reference strains, G-186B from Panama (ATCC-26030), G-217B (ATCC-26032) and Downs (ATCC-38904) from the USA. The phylogenetic species and additional details for these clinical isolates and reference strains are listed in Table 1.
Histoplasma capsulatum clinical isolates and reference strains studied.
| Isolate/strain | Year of Isolation | Phylogenetic Species/Lineage | Geographic origin | Histoplasmosis clinical form | Patient immune-compromise |
|---|---|---|---|---|---|
| EH-316 | 1993 | – | MX | D | HIV+ |
| EH-317 | 1992 | – | MX | D | HIV+ |
| EH-319 | 1991 | – | MX | D | HIV+ |
| EH-323 | 1993 | LAm A | MX | D | HIV+ |
| EH-324 | 1994 | LAm A | MX | D | HIV+ |
| EH-325 | 1996 | – | MX | D | HIV+ |
| EH-326 | 1996 | LAm A | MX | D | HIV+ |
| EH-328 | 1991 | LAm A (LAm A1) | MX | D | HIV+ |
| EH-355 | 1996 | LAm A | MX | D | HIV+ |
| EH-356 | 1996 | LAm A | MX | D | HIV+ |
| EH-359 | 1995 | – | MX | DML | Unknown |
| DMic993444 | 1999 | LAm B (LAm B1) | AR | DML | Unknown |
| DMic993445 | 1999 | LAm B (LAm B1) | AR | DML | Unknown |
| DMic993446 | 1999 | LAm B (LAm B1) | AR | DML | HIV+ |
| DMic993267 | 1999 | LAm B (LAm B1) | AR | DML | HIV+ |
| DMic01739 | 2001 | LAm B | AR | DML | HIV+ |
| LA | 1996 | LAm A (LAm A2) | CO | D | HIV+ |
| AP | – | LAm A (LAm A1) | CO | D | Unknown |
| WE | 1996 | – | CO | P | HIV+ |
| MZ 2 | 1995 | LAm A (LAm A2) | CO | DML | SLE |
| GeM | 2001 | LAm A (LAm A1) | CO | Unknown | Unknown |
| H.1.02.W | 1995 | LAm A (LAm A2) | GT | P | Unknown |
| G-186Bd,a | 1967 | H81 lineage | PA | P | Unknown |
| G-217Be,b | 1973 | NAm 2 | USA | Unknown Unknown | |
| Downsf,c | 1969 | NAm 1 | USA | DML | RA |
AR, Argentina; CO, Colombia; GT, Guatemala; MX, Mexico; PA, Panama; USA, United States of America; D, disseminated; DML, disseminated with mucosal lesions; P, pulmonary; HIV, human immunodeficiency virus; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis.
The listed H. capsulatum phylogenetic species were based on the classification by Kasuga et al.;13 in parenthesis, those lately renamed by Teixeira et al.25
All isolates studied were well characterized prior to their deposition in the H. capsulatum Culture Collection of the Fungal Immunology Laboratory of the Department of Microbiology and Parasitology, School of Medicine, UNAM (LIH-UNAM). H. capsulatum specimens in the collection were well preserved in sterile distilled water and on Sabouraud-agar with mineral oil, from the time of their first isolation. This collection is registered in the World Data Center on Microorganisms (WDCM) database of the World Federation for Culture Collections as WDCM817 LIH-UNAM and is available at http://www.wfcc.info/ccinfo/index.php/strain/display/817/fungi/.
Morphotype transitionThe M-phase of each H. capsulatum isolate and reference strain was initially cultured at 25–28 °C on mycobiotic-agar (Bioxon, Becton-Dickinson, Mexico City, MX). Mycelia were harvested by centrifuging the culture at 800 g for 15 min and then were grown in a synthetic medium18 at 37 °C in an orbital shaker at 200 rpm. This medium was replaced every 72 h for each culture until the Y-transition was reached in all cultures, in approximately one to two weeks. Once the dimorphic transition was complete, each yeast culture was incubated at 37 °C for 24–48 h in brain-heart infusion (BHI)-broth (Bioxon) supplemented with 0.1% L-cysteine and 1% glucose. The yeast cells were harvested by centrifuging at 800 g for 15 min, washed twice with fresh BHI-broth, and stored at –80 °C in the presence of fetal calf serum (GIBCO, Grand Island Biological Co. NY, USA) and dimethyl sulfoxide at a 9:1 ratio until required.
Yeast-morphotype thermosensitivityAfter gradual defrosting, the yeasts were grown in supplemented BHI-broth. Each yeast culture was incubated at 37 °C for 24–48 h, which is the estimated time required to reach the logarithmic growth phase (log-phase). Each yeast culture in the log-phase was transferred to fresh supplemented BHI-broth and incubated at 37 °C for 24 h. The yeasts were centrifuged at 800 g for 15 min, and the pellet was used for assays. To yeasts were suspended in 10 mL of supplemented BHI-broth to reach the desired inoculum of 0.2 optical densities (OD). Each yeast suspension was initially diluted 1:10 and subsequently serially diluted in 200 µL of BHI-broth of each 96-well microplate (Nunc, Roskilde, Denmark). Because the first microplate column was filled only with 200 µL of supplemented BHI-broth (blank), yeast serial dilutions were made from the second to the last columns. The OD values were measured at 405 nm using a Labsystems Multiskan MS reader (Labsystems, Helsinki, Finland), and each value was automatically adjusted with the blank. To perform the growth curve assay for each isolate and reference strain, eight wells per microplate column were filled with 200 µL of a 0.2 OD yeast dilution. Each growth curve was set up in triplicate either at 37 or 40 °C. Optical density readings were taken at 0, 3, 6, 12, 24, and 30 h of incubation at both temperatures, and the OD values were averaged using n = 12 per isolate or strain value for each time tested. The results were plotted as OD versus incubation times at both temperatures tested.
The growth inhibition percentage (GI%) at 40 °C of each H. capsulatum yeast culture was estimated from the data of the growth curve based on the following equation: GI% = 1 - (OD tx – OD t0 at 40 °C/OD tx – OD t0 at 37 °C) x 100, where tx is the OD value at each time tested and t0 represents time zero.
The doubling time (Dt) values at both 37 and 40 °C were calculated according to the following equation: Dt = OD t0/µ, where µ = OD tx on the slope of the growth curve for each isolate and reference strain studied.19
StatisticsThermosensitivity data were statistically analyzed using Student's t test (Microsoft Excel 2013 for Windows). For all analyses, means ± standard deviations (SD) were determined. A significant difference was considered when p-value ≤ 0.01.
ResultsMost of the studied H. capsulatum clinical isolates were obtained from 1991 to 2001 and belong to the Latin American group A (LAm A1 and LAm A2) and Latin American group B (LAm B1) phylogenetic species (Table 1). The reference strains G-186B, G-217B, and Downs were isolated in the 1960s and 1970s and belong to the H81 Panama lineage, North American Class 2 (NAm 2), and North American Class 1 (NAm 1) phylogenetic species, respectively (Table 1).
Of the 25 H. capsulatum yeast culture samples studied, 20 were from patients with the disseminated clinical form of histoplasmosis (eight were from patients with mucosal lesions), three derived from patients with localized pulmonary histoplasmosis, and the GeM isolate from Colombia as well as the G-217B reference strain from the USA were not associated with any special data regarding the clinical form of the respective patients. In addition, 15 isolates from Latin America (10 from Mexico, three from Argentina, and two from Colombia) came from patients infected with human immunodeficiency virus, one isolate was from a Colombian patient with systemic lupus erythematosus, and the other was the Downs reference strain from the USA originally isolated from an 86-year-old patient with rheumatoid arthritis. Finally, six isolates (one from Mexico, two from Argentina, two from Colombia, and one from Guatemala) and two reference strains (G-217-B from USA and G-186B from Panama) were derived from patients without any clinical records suggesting an immunosuppressive condition (see Table 1).
Regarding thermosensitivity detection, the H. capsulatum Y-phase of each isolate and reference strain was analyzed according to its growth curve at 37 and 40 °C, GI% at 40 °C, and Dt at 37 and 40 °C.
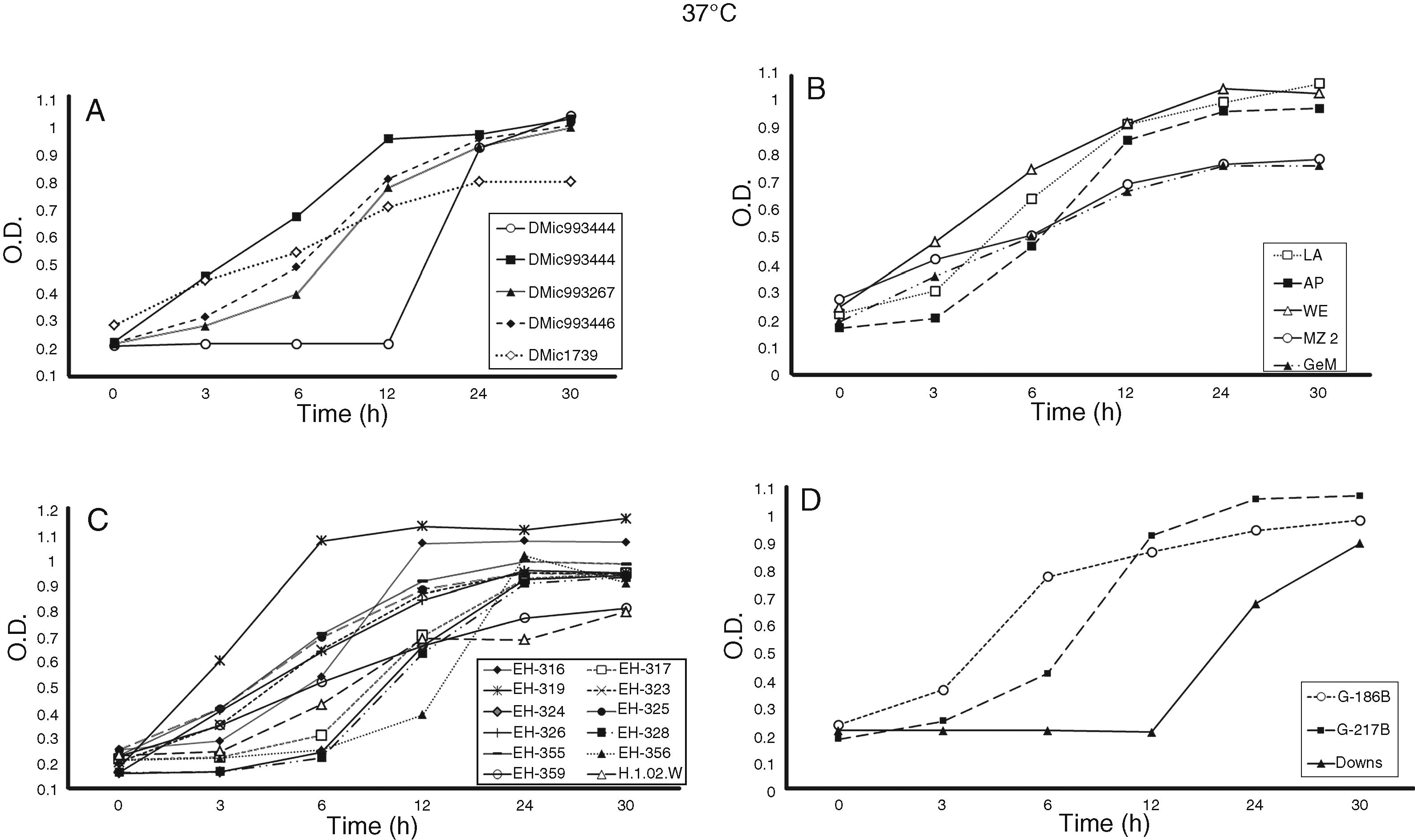
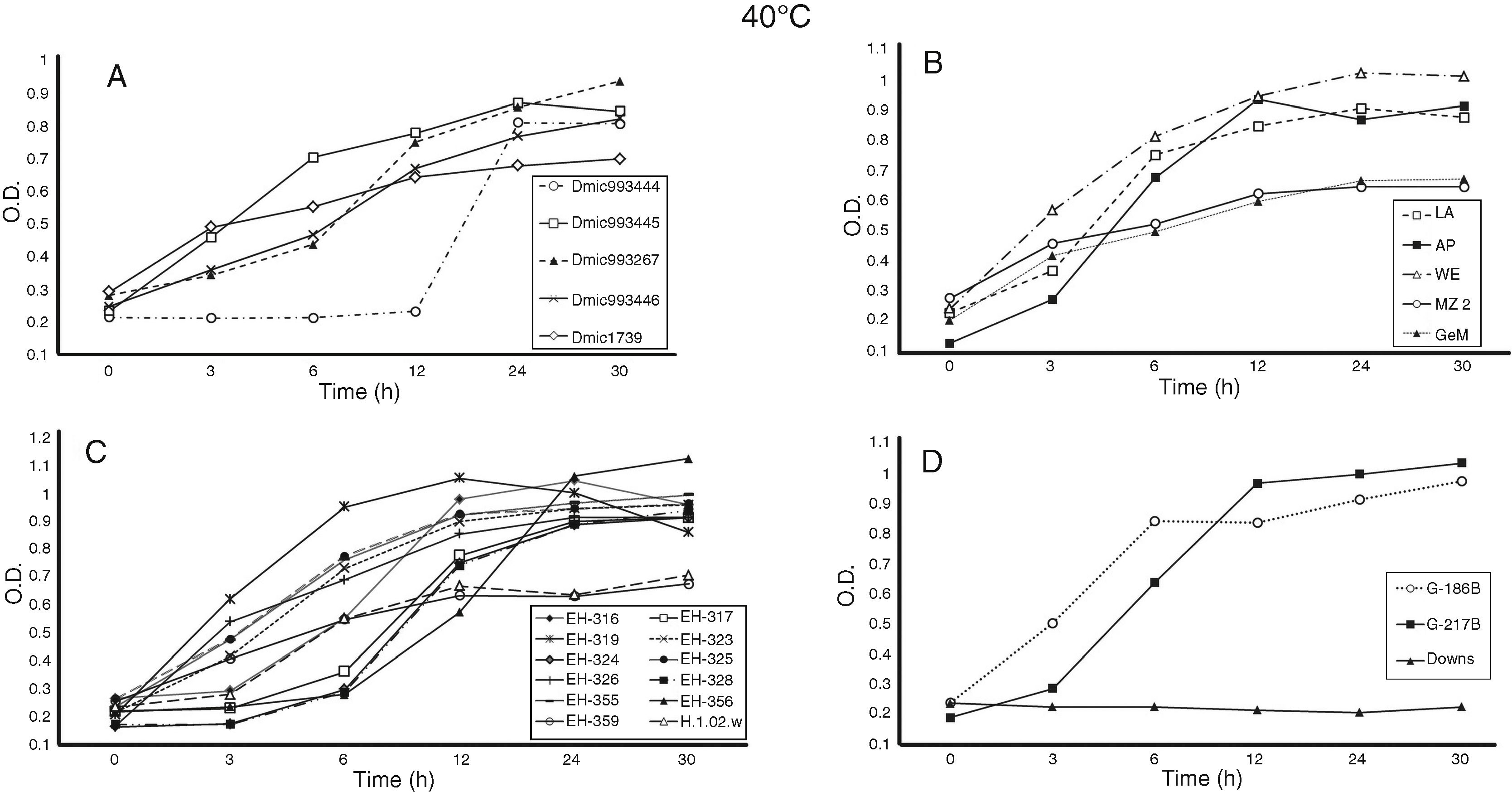
For the growth curve (Fig. 1A–D and Fig. 2A–D), all OD data at 0 h of incubation (t0) were recorded in the range of 0.164 ± 0.002 and 0.286 ± 0.001 at 37 °C and 0.129 ± 0.006 and 0.288 ± 0.001 at 40 °C (data not shown). This ensured that the initial yeast-population at t0 for all H. capsulatum isolates and reference strains studied were as homogeneous as possible. The SD confirmed the minimal variation of readings within the same population.
Growth curves of Histoplasma capsulatum yeasts at 37 °C. The OD at 405 nm for each incubation time was measured using a Multiskan reader; the yeast growth conditions and growth curve construction are described in the Material and Methods section. (A) Isolates from Argentina; (B) isolates from Colombia; (C) isolates from Mexico and Guatemala; and (D) reference strains. On the basis of three independent assays (n = 12 per OD value), significant differences in growth curves were recorded for p ≤ 0.01 along the incubation time points tested.
Growth curves of Histoplasma capsulatum yeasts at 40 °C. The OD at 405 nm for each incubation time was measured using a Multiskan reader; the yeast growth conditions and growth curve construction are described in the Material and Methods section. The isolates and reference strains analyzed were the same as described in Fig. 1.
Differences in lag (latency), log (exponential), stationary, and decline H. capsulatum growth curve phases were detected at 37 and 40 °C for the incubation time points assessed, as shown in Fig. 1A–D and Fig. 2A–D, respectively.
Most of the isolates and reference strains exhibited similar growth curves at both 37 (Fig. 1A–D) and 40 °C (Fig. 2A–D). Several isolates from Mexico, Argentina, and Colombia did not exhibit a lag-phase at 37 °C (Fig. 1A–D), reaching the log-phase before three hours and the stationary-phase between six and 24 h. In contrast, the lag-phase was well defined in the DMic993444 isolate from Argentina, four isolates from Mexico (EH-317, EH-324, EH-328, and EH-356), and the Downs strain from the USA (Fig. 1A, C, D). The DMic993444 isolate from Argentina and the Downs strain reached the log-phase after a 12 h lag-phase (Fig. 1A, D). Most isolates reached the stationary-phase at 30 h of culture, without evidence of a decline in the population.
Based on growth curves at 37 and 40 °C (Fig. 1A–D and Fig. 2A–D), all isolates and two reference strains (G-217B and G-186B) were considered thermotolerant, irrespective of the clinical form and immune condition of the patient from whom they were isolated. The Downs strain was considered thermosensitive because it did not grow at 40 °C, as shown in Fig. 2D. Differences in growth curves at 37 °C between the reference strains G-217B (thermotolerant prototype at 40 °C) and Downs (thermosensitive prototype at 40 °C) were significant (p ≤ 0.01) at 6, 12, 24, and 30 h of incubation, whereas differences in the growth curves of these strains at 40° C were not applicable due to lack of growth of the latter strain at this temperature.
The growth curve at 37 °C for the isolate DMic993444 from an Argentinean patient is noteworthy, because it showed significant differences (p ≤ 0.01) from the G-217B strain at 6–30 h and from the Downs strain at 24 and 30 h of incubation (Fig. 1A, D). In contrast to the Downs reference strain, isolate DMic993444 also exhibited growth at 40 °C after 24 and 30 h of incubation; thus, it was considered to have a thermotolerant phenotype. However, this isolate presented significant differences (p ≤ 0.01) in OD values from three to 30 h of incubation at 40° C when compared with the thermotolerant prototype G-217B reference strain (Fig. 2A, D).
Twelve H. capsulatum isolates (three from Mexico, five from Argentina, three from Colombia, and one from Guatemala) displayed GI values from 15.6 to 33.8% at 30 h of incubation at 40 °C (Table 2). The estimation of GI% at 40 °C corroborated the thermosensitivity characteristics of the isolates and reference strains studied. With the exception of the Downs strain, a thermotolerant phenotype was confirmed for all the H. capsulatum isolates and remaining reference strains because they exhibited low GI values, below 33.8% (Table 2).
Growth inhibition percentage (GI%) of Histoplasma capsulatum yeasts at 40 °C.
| Isolate/Strain | GI%a | ||||
|---|---|---|---|---|---|
| 3 h | 6 h | 12 h | 24 h | 30 h | |
| EH-316 | 2.5 | 0.7 | 12.7 | 5.7 | 15.6 |
| EH-317 | 0.0 | 0.0 | 1.4 | 5.0 | 6.2 |
| EH-319 | 2.8 | 17.1 | 11.4 | 15.7 | 33.8 |
| EH-323 | 0.0 | 0.0 | 0.0 | 2.7 | 0.7 |
| EH-324 | 0.0 | 0.0 | 0.0 | 5.9 | 4.3 |
| EH-325 | 0.0 | 0.0 | 0.0 | 2.8 | 2.8 |
| EH-326 | 0.0 | 0.0 | 0.0 | 6.4 | 5.6 |
| EH-328 | 0.0 | 0.0 | 0.0 | 4.7 | 0.9 |
| EH-355 | 0.0 | 0.0 | 0.3 | 4.8 | 4.8 |
| EH-356 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| EH-359 | 0.0 | 0.7 | 14.1 | 31.9 | 28.7 |
| DMic993444 | NA | NA | NA | 16.5 | 28.6 |
| DMic993445 | 5.0 | 0.0 | 25.7 | 15.2 | 24.2 |
| DMic993446 | 0.0 | 18.5 | 28.3 | 28.8 | 26.6 |
| DMic993267 | 3.1 | 12.3 | 16.5 | 19.0 | 15.9 |
| DMic01739 | 0.0 | 0.4 | 18.0 | 25.3 | 21.2 |
| LA | 0.0 | 0.0 | 10.8 | 12.5 | 23.2 |
| AP | 0.0 | 0.0 | 0.0 | 6.7 | 2.2 |
| WE | 0.0 | 0.0 | 0.0 | 2.5 | 1.8 |
| MZ 2 | 0.0 | 0.0 | 17.7 | 25.1 | 27.9 |
| GeM | 0.0 | 5.1 | 16.9 | 18.6 | 17.7 |
| H.1.02.W | 0.0 | 0.0 | 6.7 | 12.4 | 17.5 |
| G-186B | 0.0 | 0.0 | 5.7 | 5.2 | 1.9 |
| G-217B | 0.0 | 0.0 | 0.0 | 8.2 | 5.3 |
| Downs | NA | NA | NA | NA | NA |
NA, not applicable.
The Dt values, calculated at both 37 and 40 °C for each H. capsulatum isolate and reference strain, varied at 37 °C from 1.36 h (EH-319, Mexican isolate) to 8.30 h (MZ 2, Colombian isolate) with a mean of 5.14 h ± 1.47 h, and at 40 °C from 1.54 h (EH-319, Mexican isolate) to 9.42 h (DMic01739, Argentinean isolate) with a mean of 5.55 h ± 1.87 h (Table 3).
Doubling times (Dt) of Histoplasma capsulatum yeasts at 37 and 40 °C.
| Isolate/Strain | Dt (h)a | Changes of Dt from 37 to 40°C | |
|---|---|---|---|
| 37°C | 40°C | ||
| EH-316 | 4.36 | 5.00 | Increase |
| EH-317 | 5.12 | 6.00 | Increase |
| EH-319 | 1.36 | 1.54 | Increase |
| EH-323 | 4.06 | 5.18 | Increase |
| EH-324 | 4.30 | 4.36 | Increase |
| EH-325 | 5.00 | 6.24 | Increase |
| EH-326 | 3.18 | 3.18 | NC |
| EH-328 | 4.42 | 4.48 | Increase |
| EH-355 | 4.24 | 5.36 | Increase |
| EH-356 | 5.12 | 4.48 | Decrease |
| EH-359 | 6.54 | 8.54 | Increase |
| DMic993444 | 6.06 | 6.42 | Increase |
| DMic993445 | 5.18 | 4.54 | Decrease |
| DMic993446 | 6.36 | 7.24 | Increase |
| DMic993267 | 5.36 | 7.36 | Increase |
| DMic01739 | 6.42 | 9.42 | Increase |
| LA | 5.18 | 4.42 | Decrease |
| AP | 4.24 | 3.18 | Decrease |
| WE | 5.42 | 4.24 | Decrease |
| MZ 2 | 8.30 | 7.42 | Decrease |
| GeM | 5.00 | 7.06 | Increase |
| H.1.02.W | 7.24 | 8.00 | Increase |
| G-186B | 5.42 | 5.48 | Increase |
| G-217B | 3.30 | 4.24 | Increase |
| Downs | 7.36 | NA | NA |
| Meansb | 5.14 ± 1.47 | 5.55 ± 1.87 | |
NC, No changes; NA, Not applicable.
Two isolates (EH-319 and EH-326) from Mexican patients with Aids-associated disseminated histoplasmosis presented the fastest Dt, with values lower than the averages of all Dt values at 37 and 40 °C. In contrast, four isolates from patients with mucosal lesions (EH-359 from Mexico, DMic993446 and DMic01739 from Argentina, and MZ 2 from Colombia) and one isolate associated with a pulmonary clinical form (H.1.02.W from Guatemala) were considered to present the most delayed Dt, showing Dt mean values higher than those found at 37 and 40 °C for most of the isolates and reference strains studied (Table 3). Notably, isolate DMic993444 (Argentina) exhibited Dt values close to the Dt means obtained for H. capsulatum at both temperatures (Table 3), despite its very long lag-phase (Figs. 1A and 2A). The Dt calculated for the Downs strain at 37 °C was among the longest values obtained in this study (Table 3); due to its thermosensitive phenotype data at 40 °C were not obtained for the Downs strain.
Of the 25 isolates and reference strains of H. capsulatum studied, 15 isolates and two reference strains showed increase in Dt from 37 to 40 °C, six isolates exhibited decreased Dt, and one isolate maintained the same Dt value at both temperatures, whereas the Downs strain did not grow at 40 °C as mentioned above (see details in Table 3).
DiscussionIt is important to note that all H. capsulatum clinical isolates selected for this study have a limited number of subcultures in suitable media after their first isolation. In addition, because they were taken from their original cultures, as explained in the Material and Methods section, they did not develop critical phenotypic changes. Conversely, the reference strains G-186B, G-217B, and Downs have been cultured repeatedly.
Some phenotypic characteristics (e.g., serotypes, chemotypes, isozymes and fatty acid profiles) of the two H. capsulatum morphotypes have been explored to group and/or classify fungal strains.7,9,20,21 In a few cases, these data were analyzed by overlooking important associations with critical aspects of the interaction between the fungus and its environment. Studies of H. capsulatum assessing thermosensitivity associated with fungal virulence were conducted by Medoff et al.,6 Keath et al.,22 Spitzer et al.,11 and Gargano et al.23 Recently, Garfoot et al.24 suggested that the thermotolerance of the Histoplasma Y-phase to the body temperature of the mammalian host is associated with the O-mannosylation of its proteins. However, most of these reports evaluated a scarce number of H. capsulatum isolates or strains that came from circumscribed geographic areas from North America, which are not representative of the genetic and phenotypic fungal changes that may be occurring in different regions associated with the phylogeographic distribution of the H. capsulatum complex in the Americas, as stated by Kasuga et al.13 and Teixeira et al.25
The present work represents an innovative report because all the studied H. capsulatum clinical isolates were from Latin American countries, where this type of phenotype evaluation had not been described until now. Based on limited subcultures of these isolates, their thermo-phenotypic characteristics can be considered to be naive. The data obtained for the 22 isolates and three strains studied are reliable as they incorporated the results of multiple measurements in repetitive assays. All H. capsulatum isolates and two reference strains studied were thermotolerant at 40 °C. The exception was the Downs reference strain, confirming its thermosensitive phenotype at this temperature. Interestingly, the Downs strain is also associated with avirulence in a murine model, and according to Spitzer et al.,11 a potential association between virulence and thermotolerance in the H. capsulatum Y-phase can be inferred. However, no associations between H. capsulatum temperature sensitivity and the clinical forms of histoplasmosis or the immune status of the patients were found.
Regarding Dt estimation, we found delayed Dts at 40 °C for most of the studied H. capsulatum samples, indicating that fungal exposure to this temperature delayed the growth of thermotolerant yeasts, but did not kill them. Under in vivo infection conditions, this finding suggests that host defense mechanisms might include the restriction of fungal dissemination via febrile inflammatory processes. The Dt values at 37 °C found for most studied isolates and reference strains were compatible for pathogens with a low rate of growth and consistent with previous descriptions for H. capsulatum.5,15
Interestingly, the isolate DMic993444 obtained from an Argentinean histoplasmosis patient with mucosal lesions associated with a disseminated clinical form, presented a long lag-phase at both 37 and 40 °C, suggesting a probable delay in adaptation to the experimental conditions.
Despite the involuntary biases that must be considered in the gathering and handing of patient records, the present findings provide relevant information regarding the thermo-phenotypic characteristics of the H. capsulatum Y-phase, irrespective of associations with clinical disease forms and different geographic origins in the Americas. An appropriate number of fungal isolates and reference strains were tested, and, undoubtedly, this type of study will advance the knowledge on the possible manifestations of the pathogen in susceptible hosts.
ContributionsMLT and MRRM were involved in the study design, analyzed and interpreted the results and drafted the manuscript. JHS performed the experiments, conducted the data analyses and participated in drafting the manuscript. GRA maintained the fungal cultures. CC provided a critical review of the manuscript. MLT conceptualized and coordinated the project. All authors read and approved the final manuscript.
FundingThis research was funded by a grant from the National Council of Science and Technology (CONACYT) from Mexico (Ref-166052).
Conflicts of interestThe authors declare no conflicts of interest.
This paper constitutes collateral fulfillment of the requirements of the Graduate Program in Biological Sciences of the UNAM. JHS thanks the Graduate Program in Biological Science of the UNAM and the scholarship of CONACYT-Mexico (Ref-245151).