Influenza continues to drive seasonal morbidity, particularly in settings with low vaccine coverage.
ObjectivesTo describe the influenza cases and viral circulation among hospitalized patients.
MethodsA prospective study based on active surveillance of inpatients with influenza-like illness from a tertiary hospital in Bucharest, Romania, in the season 2016/17.
ResultsA total of 446 patients were tested, with a balanced gender distribution. Overall, 192 (43%) patients tested positive for influenza, with the highest positivity rate in the age groups 3–13 years and >65 years. Peak activity occurred between weeks 1 and 16/2017, with biphasic distribution: A viruses were replaced by B viruses from week 9/2017; B viruses predominated (66.1%). Among the 133 (69.3%) subtyped samples, all influenza A were subtype H3 (n=57) and all influenza B were B/Victoria (n=76). Patients who tested positive for influenza presented fewer comorbidities (p=0.012), except for the elderly, in whom influenza was more common in patients with comorbidities (p=0.050). Disease evolution was generally favorable under antiviral treatment. The length of hospital stay was slightly longer in patients with influenza-like illness who tested patients negative for influenza (p=0.031).
ConclusionsDistinctive co-circulation of A/H3 and B/Victoria in Bucharest, Romania in the 2016/17 influenza season was found. While the A/H3 subtype was predominant throughout Europe that season, B/Victoria appears to have circulated specifically in Romania and the Eastern European region, predominantly affecting preschoolers and school children.
Influenza continues to be a major cause of seasonal morbidity and mortality. Surveillance of the circulation of different influenza strains is important in ensuring a good strain concordance for the composition of the annual influenza vaccine. In Romania, influenza vaccination is available to everyone above the age of 6 months, but it is provided free-of-charge by the Ministry of Health only for risk groups such as patients with comorbidities, HIV infection, pregnant women, persons over 65 years of age, institutionalized persons, and social and healthcare workers. In the season 2016/17 the overall influenza vaccine uptake in the general population was very low, 2.5%, according to the Romanian National Center for Surveillance and Control of Transmissible Diseases. The low uptake for the 2016/17 season was similar with that from the past influenza seasons, when the uptake was 3.2%, 2.5%, 2.7%, 4.2%, and 3%, respectively.1 The vaccine uptake progressively decreased in the past 10 years, since the 2007/08 season when an uptake of 16.6% was reported.2
In this context, we conducted a prospective study aimed at characterizing severe influenza disease, and describing the circulation of the different influenza types among hospitalized cases admitted to a tertiary hospital from Bucharest, Romania in the season 2016/17.
Material and methodsThis was a prospective study based on active surveillance of cases with influenza-like illnesses (ILI) and severe acute respiratory infections (SARI) admitted to the National Institute for Infectious Diseases “Prof. Dr. Matei Balş”, Bucharest, Romania, in the 2016/17 influenza season, from week 51/2016 to week 17/2017. Patients aged below 65 years were screened for the Global Influenza Hospital Surveillance Network (GIHSN) study, and elderly patients (age 65 years and over) were screened for the I-MOVE+ study, according to the eligibility criteria presented below.
Included patients were those with sudden onset of disease seven days prior to admission, hospitalized for at least 24h in the institute for a diagnostic compatible with ILI or SARI, defined as either:
- -
for patients below five years of age, admission diagnoses possibly associated with an influenza infection based on ICD-10 codes were used, according to the GIHSN core study protocol (www.gihsn.org)3;
- -
for patients five years of age and older, a combination of at least one of the following four systemic symptoms: fever or feverishness, headache, myalgia and malaise, and at least one of the following three respiratory symptoms: cough, sore throat, shortness of breath;
- -
in patients 65 years and older, the following criteria were also accepted as systemic symptoms suggestive for SARI: altered clinical state defined as asthenia, anorexia, confusion, weight loss.
The willingness to participate in the study was documented by signing an informed consent by the patient, or by the parents, for minors.
Institutionalized patients, non-resident or not belonging to the predefined population study base (catchment area defined as Bucharest-Ilfov area, Romania), and those with history of hospitalization in the previous 30 days were excluded.
The study protocols were approved by the Bioethics Committee of the National Institute for Infectious Diseases “Prof. Dr. Matei Balş”, Bucharest, Romania – GIHSN approval number c/7622 dated 21.11.2016 and by the Ethics Committee of the Cantacuzino Military-Medical Research-Development National Institute, Bucharest, Romania – I-MOVE+ approval number 108 dated 07.09.2016.
After receiving the informed consent from patients, the investigators checked the eligibility criteria and obtained a complete medical history. For eligible patients 14 years and over, a nasopharyngeal and a pharyngeal swab were collected, while for those <14 years old a nasopharyngeal and a nasal swab were taken, using Copan universal transport medium collection kits (Copan Diagnostics, Inc, Murrieta, CA USA).
The institute's molecular diagnosis laboratory performed real time RT-PCR Xpert Flu/RSV (Cepheid, Sunnyvale, CA, USA) to identify the presence of influenza type A or B, followed by a second rRT-PCR on the HA gene of influenza A strains (Allplex Respiratory panel 1 – Seegene, Seoul, South Korea) for determination of influenza subtypes H1, H1pdm, or H3 subtypes using the manufacturer's instructions.
A second RT-PCR analysis (SNP genotyping) of influenza B viruses was performed for determination of lineage: Yamagata or Victoria. A TAMRA probe was designed for both B virus lineages that can be detected and discriminated simultaneously, as only one of the two probes will give a fluorescent signal.4
Reaction conditions were established for the CFX96 Real-Time System (BioRad, Hercules, California, USA) in a total reaction mixture volume of 25μL using SuperScript III One-Step RT-PCR with Platinum Taq (Invitrogen, Carlsbad, CA, United States) kit with 900nM forward primer (5′-ACCCTACARAMTTGGAACYTCAGG-3′), 600nM reverse primer (5′-ACAGCCCAAGCCATTGTTG-3′), 150nM Yamagata probe TAMRA (5′-FAM-AATCCGMTYTTACTGGTAG-TAMRA-3′), 100nM Victoria probe TAMRA (5′-VIC-ATCCGTTTCCATTGGTAA-TAMRA3′) and 5μL of template RNA. Cycling conditions were 20min at 45°C and 2min at 95°C, followed by 45 cycles of 10s at 95°C and 30s at 48°C, when the fluorescent signal was captured.
We collected data on demographic, clinical and virological characteristics of ILI or SARI patients admitted to our hospital, presence of comorbidities, history of influenza vaccination during the current and previous two seasons, as well as antiviral treatment and disease evolution. For continuous variables with non-parametric distribution the median and interquartile range (IQR) are reported, and differences between groups are analyzed with the Mann–Whitney U test. For categorical variables the frequencies and percentages are reported, and differences between groups are compared with the Chi-squared test. IBM SPSS Statistics for Windows, version 20 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
ResultsStudy flowchartA total of 902 patients aged less than 65 years old were screened for the GIHSN study, and 53 patients 65 years and above for the I-MOVE+ study. Of these, 393 (43.6%) patients <65 years old met the eligibility criteria, as well as all elderly patients, making a total of 446 patients tested for the presence of influenza viruses.
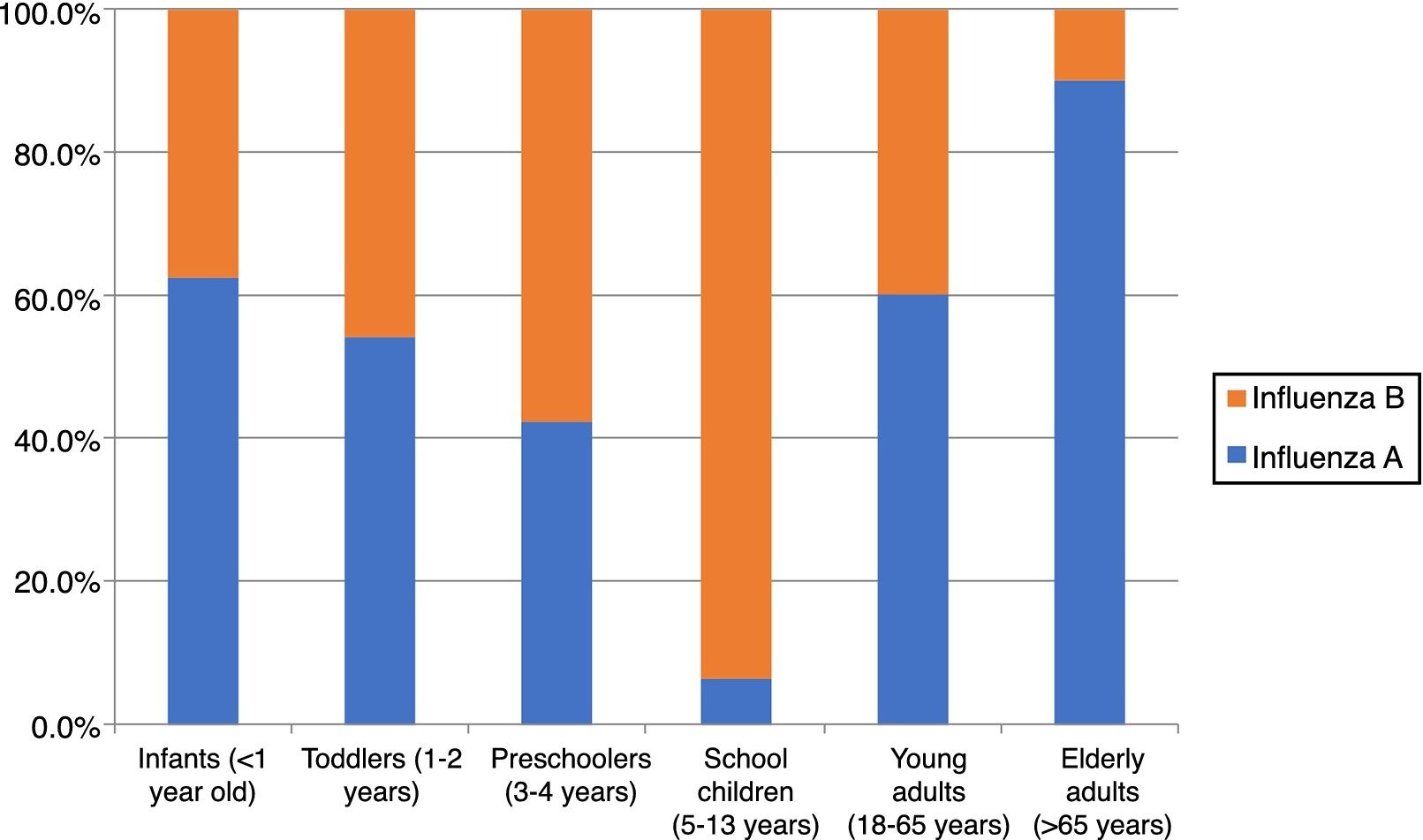
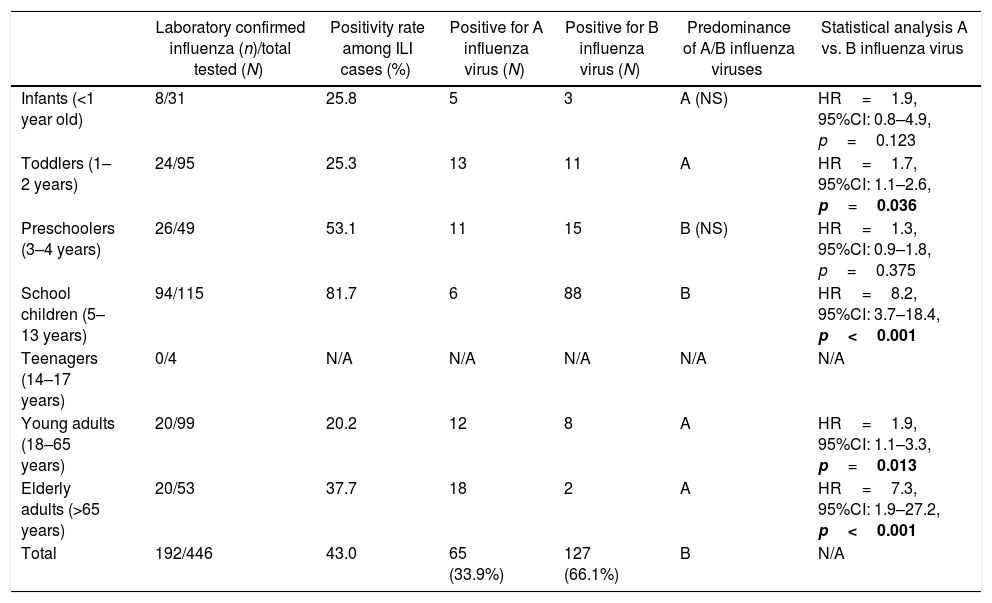
Laboratory resultsOverall, 43% (192/446) of the patients tested positive for influenza, with a similar distribution among females (39.4%) and males (46.4%), [p=0.152]. The highest influenza positivity rate was found in preschoolers, school children, and elderly patients (Table 1).
Influenza positivity rate by age group in patients hospitalized for ILI in Bucharest, Romania in the 2016/17 season.
| Laboratory confirmed influenza (n)/total tested (N) | Positivity rate among ILI cases (%) | Positive for A influenza virus (N) | Positive for B influenza virus (N) | Predominance of A/B influenza viruses | Statistical analysis A vs. B influenza virus | |
|---|---|---|---|---|---|---|
| Infants (<1 year old) | 8/31 | 25.8 | 5 | 3 | A (NS) | HR=1.9, 95%CI: 0.8–4.9, p=0.123 |
| Toddlers (1–2 years) | 24/95 | 25.3 | 13 | 11 | A | HR=1.7, 95%CI: 1.1–2.6, p=0.036 |
| Preschoolers (3–4 years) | 26/49 | 53.1 | 11 | 15 | B (NS) | HR=1.3, 95%CI: 0.9–1.8, p=0.375 |
| School children (5–13 years) | 94/115 | 81.7 | 6 | 88 | B | HR=8.2, 95%CI: 3.7–18.4, p<0.001 |
| Teenagers (14–17 years) | 0/4 | N/A | N/A | N/A | N/A | N/A |
| Young adults (18–65 years) | 20/99 | 20.2 | 12 | 8 | A | HR=1.9, 95%CI: 1.1–3.3, p=0.013 |
| Elderly adults (>65 years) | 20/53 | 37.7 | 18 | 2 | A | HR=7.3, 95%CI: 1.9–27.2, p<0.001 |
| Total | 192/446 | 43.0 | 65 (33.9%) | 127 (66.1%) | B | N/A |
HR, hazard ratio; NS, not statistically significant; 95%CI, 95% confidence interval.
Bold denotes statistically significant differences.
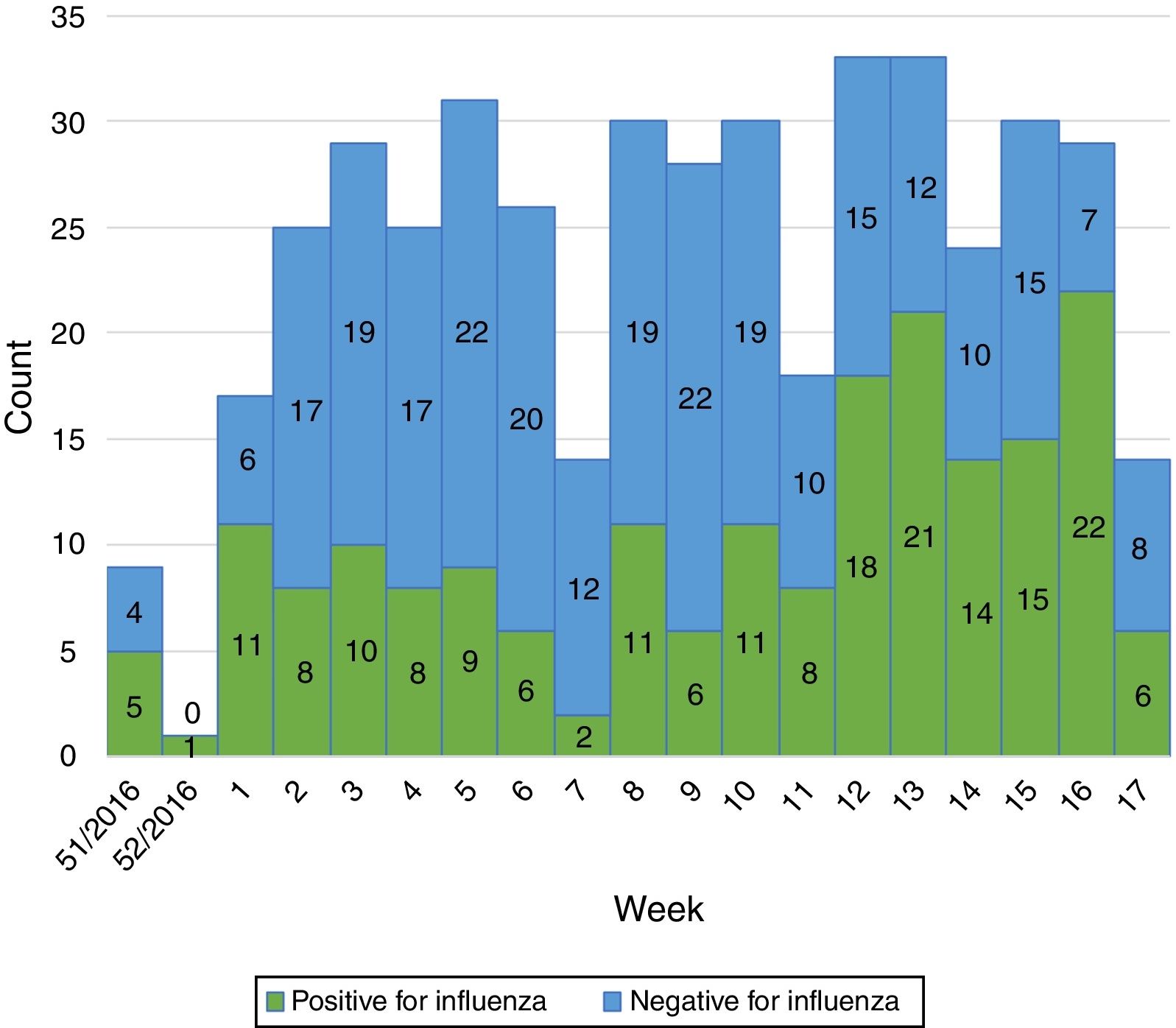
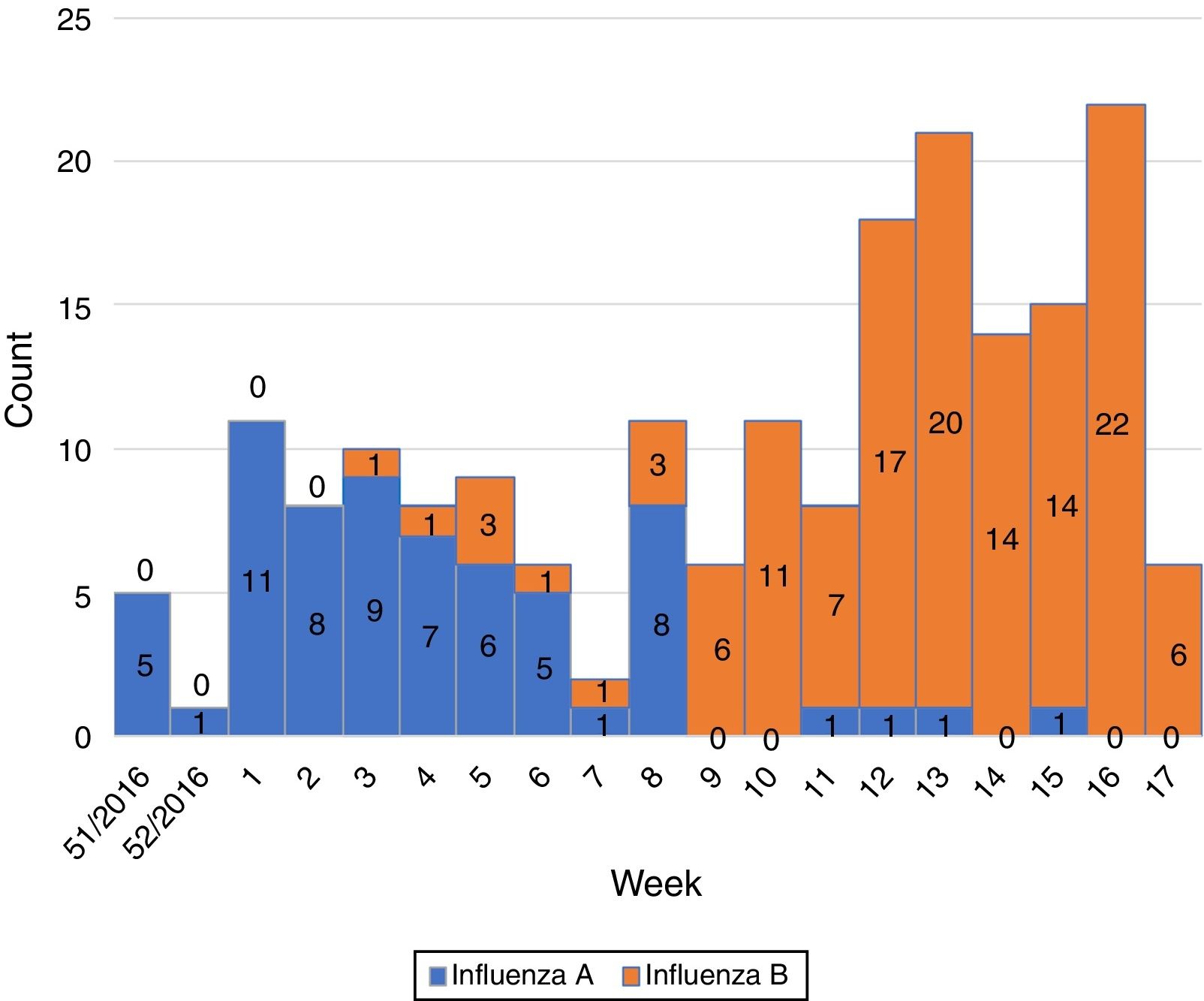
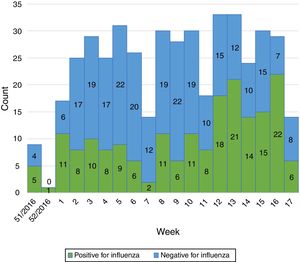
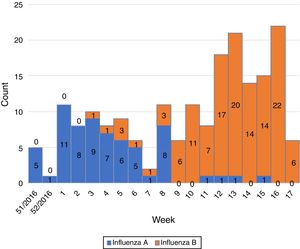
Influenza activity was recorded from week 1/2017 to week 17/2017 (Fig. 1), with influenza A predominant activity from week 1/2017 to week 8/2017, replaced by influenza B activity from week 9/2017 to week 17/2017 (Fig. 2).
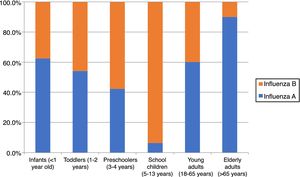
The season 2016/17 was characterized by a predominance of influenza B over A viruses in our study. Influenza B accounted for 127 (66.1%) of all laboratory-confirmed influenza cases. Cases of influenza A and B cases did not significantly differ by sex, 50.8% and 59.1% were male, respectively (p=0.286). Influenza A predominated in infants, toddlers and adults, while B viruses predominated in children aged 3–13 years (Table 1, Fig. 3).
A total of 133 (69.3%) positive samples from all study weeks were further tested to identify the subtype or lineage respectively. All subtyped influenza A samples belonged to subtype H3 (n=57) and all tested B samples belonged to B Victoria lineage (n=76).
Coinfection with influenza and respiratory syncytial virus (RSV) was detected in eight cases (three cases of influenza A and five cases of influenza B). Half of these cases occurred in children under four years of age. We also identified 32 other cases of RSV infection, in ILI patients who tested negative for influenza.
Patient characteristicsCompared to negative cases, influenza cases had similar distribution by sex (p=0.152) and age (p=0.821).
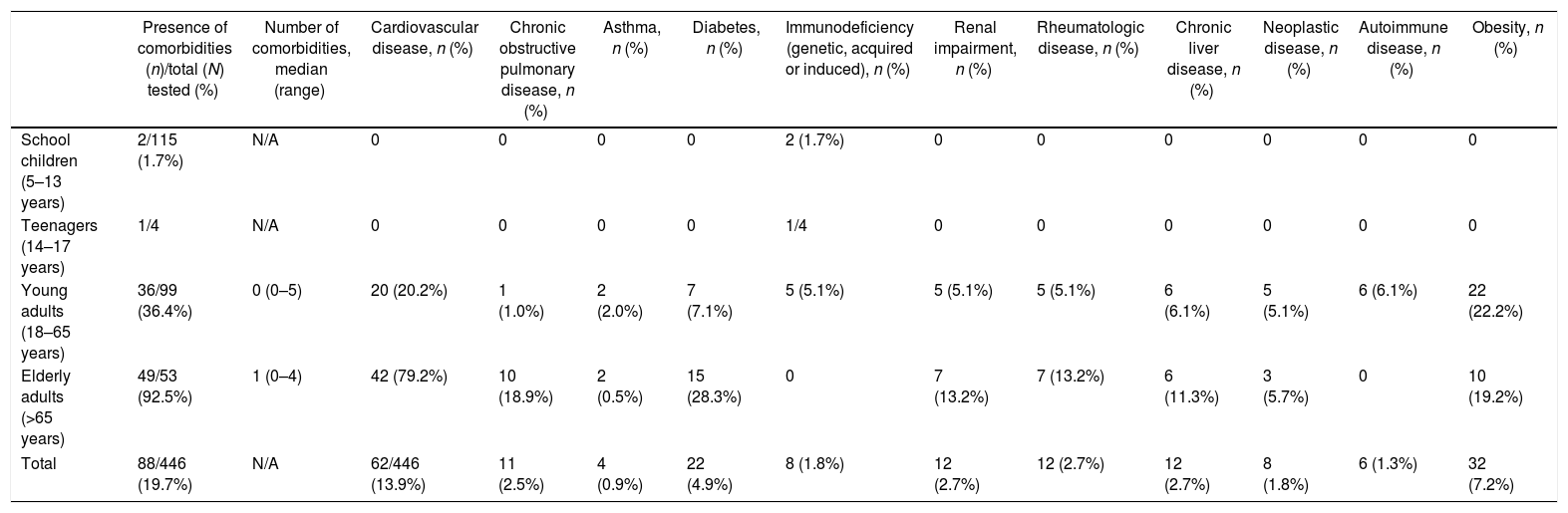
Infants, toddlers and preschoolers included in this study had no associated diseases; the proportion of comorbidities increased with age: 36 (36.4%) adults and 49 (92.5%) elderly patients presented at least one associated disease, mainly cardiovascular disease and diabetes (Table 2). None of the patients presented neuromuscular disease. Obesity was identified in 22 (22.2%) adults and 10 (19.2%) elderly patients.
Baseline characteristics of the study population by age group.
| Presence of comorbidities (n)/total (N) tested (%) | Number of comorbidities, median (range) | Cardiovascular disease, n (%) | Chronic obstructive pulmonary disease, n (%) | Asthma, n (%) | Diabetes, n (%) | Immunodeficiency (genetic, acquired or induced), n (%) | Renal impairment, n (%) | Rheumatologic disease, n (%) | Chronic liver disease, n (%) | Neoplastic disease, n (%) | Autoimmune disease, n (%) | Obesity, n (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| School children (5–13 years) | 2/115 (1.7%) | N/A | 0 | 0 | 0 | 0 | 2 (1.7%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Teenagers (14–17 years) | 1/4 | N/A | 0 | 0 | 0 | 0 | 1/4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Young adults (18–65 years) | 36/99 (36.4%) | 0 (0–5) | 20 (20.2%) | 1 (1.0%) | 2 (2.0%) | 7 (7.1%) | 5 (5.1%) | 5 (5.1%) | 5 (5.1%) | 6 (6.1%) | 5 (5.1%) | 6 (6.1%) | 22 (22.2%) |
| Elderly adults (>65 years) | 49/53 (92.5%) | 1 (0–4) | 42 (79.2%) | 10 (18.9%) | 2 (0.5%) | 15 (28.3%) | 0 | 7 (13.2%) | 7 (13.2%) | 6 (11.3%) | 3 (5.7%) | 0 | 10 (19.2%) |
| Total | 88/446 (19.7%) | N/A | 62/446 (13.9%) | 11 (2.5%) | 4 (0.9%) | 22 (4.9%) | 8 (1.8%) | 12 (2.7%) | 12 (2.7%) | 12 (2.7%) | 8 (1.8%) | 6 (1.3%) | 32 (7.2%) |
Note: None of the infants (<1 year old), toddlers (1–2 years) and preschoolers (3–4 years) presented any of the listed comorbidities.
There was no statistical association between presence of comorbidities and positivity for influenza in the 18–65 years age group (p=0.796), but an overall lower number of comorbidities in patients who tested positive for influenza (p=0.012, U=21997, r=−0.1) was observed. In the elderly population however, all cases of influenza occurred in patients with comorbidities, as follows: nine patients (45% of positive cases) had one comorbid disease, six (30%) had two comorbidities, and five (25%) had three or more comorbidities. Influenza occurrence was more common in patients with comorbidities (100%) than among negative cases (82.6%), [p=0.050].
In children aged under five years, the median (IQR) gestational age at birth was 38 (37.3–39) weeks, and the median (IQR) birthweight was 3100g (2750–3450g); none of these factors were significantly associatied with the risk of influenza (p=0.695 and p=0.085). Most (82.4%) of these children had been breastfed (31.7% less than three months, 20.7% three to six months, and 47.6% over six months).
Most of the patients (or parents, for children up to 14 years old) in the study had never smoked (55.0%), while 36.9% were current smokers and 8.1% were past smokers; smoking status was not significantly associated with influenza in our patient population (p=0.206).
Among the eight cases hospitalized with influenza-like illness during pregnancy, one was confirmed with influenza A, one with influenza B, two with RSV infection, and the other five were negative for the tested viruses.
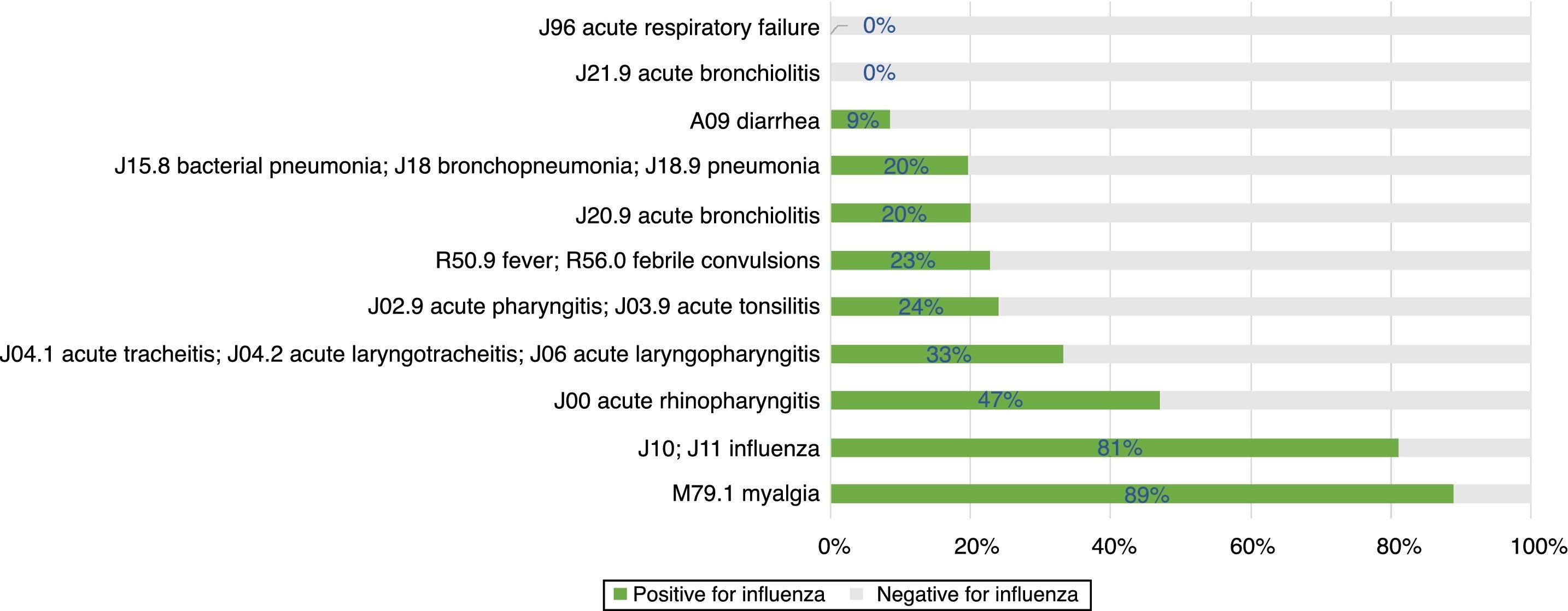
Among different diagnosis codes at admission, the highest rate of positivity for influenza was recorded for myalgia and clinical suspicion of influenza (both above 80%), followed by other diagnoses of respiratory infections, such as acute rhinopharyngitis, tracheitis, laryngotracheitis, or laryngopharyngitis (Fig. 4). None of the seven patients admitted with the diagnosis of respiratory failure tested positive for influenza, but three of them tested positive for RSV.
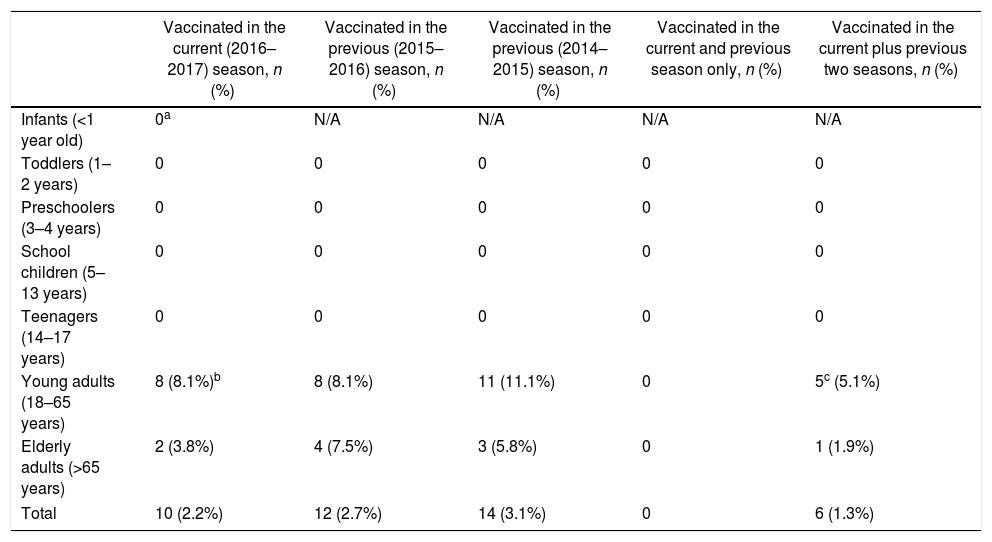
Overall, 11 (2.5%) patients had been vaccinated in the current influenza season, all of them with trivalent inactivated influenza vaccine. The vaccine brands used were: Influvac (Abbott Biologicals B.V., Olst, Netherlands) in eight patients and Vaxigrip (Sanofi Pasteur S.A., Lyon, France) in three patients. One case had been vaccinated with Vaxigrip 10 days prior to symptom onset. Among the remaining vaccinated cases, two were confirmed with influenza (both with influenza A) while eight were negative for influenza (Table 3). Due to the low number of vaccinated patients, we were unable to calculate vaccine effectiveness in this study.
Influenza vaccination coverage by age group in patients hospitalized for ILI in Bucharest, Romania in the 2016/17 season.
| Vaccinated in the current (2016–2017) season, n (%) | Vaccinated in the previous (2015–2016) season, n (%) | Vaccinated in the previous (2014–2015) season, n (%) | Vaccinated in the current and previous season only, n (%) | Vaccinated in the current plus previous two seasons, n (%) | |
|---|---|---|---|---|---|
| Infants (<1 year old) | 0a | N/A | N/A | N/A | N/A |
| Toddlers (1–2 years) | 0 | 0 | 0 | 0 | 0 |
| Preschoolers (3–4 years) | 0 | 0 | 0 | 0 | 0 |
| School children (5–13 years) | 0 | 0 | 0 | 0 | 0 |
| Teenagers (14–17 years) | 0 | 0 | 0 | 0 | 0 |
| Young adults (18–65 years) | 8 (8.1%)b | 8 (8.1%) | 11 (11.1%) | 0 | 5c (5.1%) |
| Elderly adults (>65 years) | 2 (3.8%) | 4 (7.5%) | 3 (5.8%) | 0 | 1 (1.9%) |
| Total | 10 (2.2%) | 12 (2.7%) | 14 (3.1%) | 0 | 6 (1.3%) |
Hospitalization was slightly longer in ILI patients who tested negative for influenza, with a median (IQR) of 5 (4–6) days for those with influenza (p=0.031, U=16613, r=-0.1). Patients with RSV coinfection also had a slightly longer duration of hospital stay, one extra day, compared to patients with influenza only (p=0.367).
All patients with influenza were treated with oseltamivir, with a clinical course generally favorable under treatment. Two children, one (3.8%) preschooler and one school child (1.1%), and one adult (1.0%) required intensive care and subsequently recovered. However, we recorded one death in a male toddler (4.2%), not vaccinated for influenza, with no previously documented comorbidities, who was admitted for severe clinical influenza, and confirmed with influenza A/H3, bacterial superinfection, and sepsis. No deaths were recorded in adults.
DiscussionThis study presents the characteristics of hospitalized influenza in a tertiary hospital in Bucharest, Romania, as well as the distribution of the different influenza types and subtypes.
In the EU/EEA, influenza activity of the season 2016/17 peaked from week 52/2016 to week 4/2017 and returned to baseline in week 17/2017.5 We noticed a similar start of the influenza season in our study (week 1/2017) but we also recorded prolonged influenza activity until week 17/2017. Influenza activity had a biphasic evolution this season, with A viruses predominating at the beginning of the season and being replaced by B viruses from week 9/2017 to week 17/2017. This is in line with the data reported for the entire country (replacement following week 7/2017 and continued activity until week 16/2017 on a national level).1 A recent study from France has also described this biphasic pattern, with B influenza cases peaking later than A influenza cases.6 The same study also reported a frequent circulation of B viruses in school children,6 as identified in our study.
The influenza positivity rate was highest in children aged 3–13 years admitted to the hospital for ILI, followed by 37.7% in SARI cases in elderly patients, a rate which is comparable to the 32% rate reported for the overall Romanian SARI elderly patients this season.1 The positivity rate was well below 30% in children with ages six months to three years and in young adults.
The observed occurrence of influenza in patients under 65 with an overall lower number of comorbidities in our study suggests that this season influenza viruses displayed a significant circulation in patients who were relatively healthy at baseline. This can in part be explained by the fact that, as described above, almost two thirds of the patients included in the study were children, and they did not present comorbidities. In the elderly population, we did notice a trend toward influenza occurrence in the presence of comorbidities.
In terms of circulating strains, A/H3 and B/Victoria were predominant in our study in the 2016/17 influenza season. We identified a higher number of infections with influenza B viruses, which is somewhat surprising given the data reported by the ECDC on EU/EEA level, namely that 94% of the specimens positive for influenza were type A.5 The European rate is much higher than that identified in our study (33.9% A viruses) and than that reported at the national level by the Romanian National Center for Surveillance and Control of Transmissible Diseases (65.7%).1 Two main factors could have contributed to this difference: (1) according to the study protocol, we tested only those ILI cases that required hospitalization, additional cases may have been treated by general physicians or in secondary level hospitals; (2) we tested all ILI cases in our hospital in the study period. Data reported at the national level is based on two surveillance systems, one for SARI (patients hospitalized in 20 hospitals from six counties, with one patient tested per week per hospital during the influenza season), and one for ILI (sentinel general practitioners network, with 192 general practitioners from 16 counties all over the country, testing all patients attending the general practitioner every Tuesday during the influenza season).7 Therefore, even when comparing our results to those derived from the ILI sentinel system, a substantial difference can be expected.
The circulation of A/H3 viruses identified in our study is concordant with the data reported at national level, where only one H1 virus was identified among all subtyped A viruses (n=303), the others being H3.1 This is also in line with information provided by the ECDC (98% of A viruses were subtype H3N2, this A subtype circulating almost exclusively in all reporting countries in the 2016/17 season).5
A surprising finding was that all B influenza viruses subtyped in our study belonged to the Victoria lineage, while data from the EU/EAA showed that only 20% of circulating B viruses this season were B/Victoria, most of them being B/Yamagata.5,8 Furthermore, both Victoria and Yamagata lineages have been shown to circulate simultaneously in previous influenza seasons, in countries such as Brazil.9 Lineage data for B viruses has not been reported at the national level in Romania this season and therefore our findings fill a gap in current knowledge, showing that B/Victoria was the main circulating B subtype, an apparently different pattern compared to that reported in the rest of Europe in the same influenza season. This can in part be explained by the predominance of B influenza in children in our study; a recent study has indeed shown that among B influenza, lineage B/Victoria was more frequently identified in children during the past influenza seasons.10 Circulation of A/H3 and B/Victoria this season was also reported by neighboring Bulgaria,11 and our results are also in line with publicly-available data from the GIHSN network, which showed B/Victoria lineage also as predominant among B influenza viruses this season in countries such as Russia,12,13 Ivory Coast,14 Kazakhstan,15 India,16 and China.17
We have reported a low vaccination uptake (2.5%) in patients admitted to the hospital with ILI in Bucharest Romania during the influenza season 2016/17. This highlights the need for a better vaccine uptake, both for specific risk groups and the general population, given the availability of seasonal vaccines and the new options in the pipeline.18–20
The present study has also drawn attention to the mixed viral respiratory infections, with RSV coinfecting 4.2% of the cases of influenza. The important interactions of influenza A viruses with bacterial pathogens have previously been described,21–23 and we are now briefly describing the burden of viral coinfection. A recent study from Madagascar has also reported a relatively high rate of influenza and RSV coinfection (10.6%) in hospitalized patients, and that RSV infection was associated with longer hospitalization,24 while a lower rate of coinfection was reported in the UK (1.3% for influenza A/H1N1pdm09 and RSV, and 2.7% for seasonal influenza and RSV).25 However, as our study period did not cover the whole RSV season, we aim to perform a future systematic screening study for RSV during the cold season, to further describe the RSV infection in patients with ILI, particularly since the circulation of RSV has been shown to also display changing patterns in terms of seasonality.26
The study had some limitations: one of them is related to the high number of children included, which probably led to a slight overrepresentation of the percentage of B virus infections. However, the sampling strategy reflected the overall predominance of influenza cases in children this season in the study area, as well as their higher addressability to the hospital, compared to adults, who might have also been treated by general practitioners, or as outpatients. Another limitation is that the population study base consisted of only one hospital, which is a reference center for infectious diseases in Romania and also receives severe cases from all over the country. As the catchment area was defined in the GIHSN study protocol as the Bucharest-Ilfov area only, cases of ILI that occurred in non-residents were excluded from the study, leading to lower sampling rates in weeks when outbreaks of influenza occurred in other regions of the country, and non-resident patients were referred to our hospital, as in weeks 7/2017 and 11/2017. Our institute's status as a reference center is also associated with the somewhat high rate of influenza positivity in our study, compared with what is typically reported in an influenza season; this mainly derives from the study eligibility criteria, namely the definition of a “severe” case of influenza as a hospitalized case. As the institute is a reference center in the city and in the country, one might expect that patients with a high clinical index of suspicion for influenza be admitted more often compared to other cases of milder viral respiratory tract infections. A third limitation is that we were unable to provide a robust estimate for vaccine effectiveness, given the low number of patients who had been vaccinated in the present study (n=10). Finally, the slight differences in the case definitions for influenza-compatible illness used for children <5 years old, patients >5 years old and elderly patients, may impact to some extent the comparability of positivity rates between these age categories. This study also has a number of strengths, as it provides the first robust characterization of influenza disease in Bucharest Romania and is the first report on the co-circulation of A/H3 and B/Victoria subtypes in Romania in 2016/17.
ConclusionsWe report a distinct co-circulation of A/H3 and B/Victoria in Bucharest, Romania in the 2016/17 influenza season, which occurred mainly in previously healthy young patients and in elderly patients with comorbidities. While the A/H3 subtype was predominant throughout Europe this season, B/Victoria appears to have circulated specifically in Romania and possibly other Eastern European countries, predominantly affecting preschoolers and school children. Influenza activity presented a biphasic evolution, the peak for A viruses preceding the peak for B viruses by approximately eight weeks. Continued surveillance of influenza and potentially other respiratory viruses is necessary to inform local and regional policies.
Disclosure and competing interest statementACD: Manager of the GIHSN project funded by Sanofi Pasteur and Foundation for Influenza Epidemiology; no conflict of interest.
OS: Member of the research team of the GIHSN project funded by Sanofi Pasteur and Foundation for Influenza Epidemiology; subinvestigator in influenza clinical trials by Activaero and Shionogi; no conflict of interest.
DF: Member of the research team of the GIHSN project funded by Sanofi Pasteur and Foundation for Influenza Epidemiology; no conflict of interest
OV: Member of the research team of the GIHSN project funded by Sanofi Pasteur and Foundation for Influenza Epidemiology; no conflict of interest.
ASC: Subinvestigator in influenza clinical trials by Activaero and Shionogi; no conflict of interest.
DO: Member of the research team of the GIHSN project funded by Sanofi Pasteur and Foundation for Influenza Epidemiology; no conflict of interest.
VA: Member of the research team of the GIHSN project funded by Sanofi Pasteur and Foundation for Influenza Epidemiology; no conflict of interest.
MLL: Member of the research team of the GIHSN project funded by Sanofi Pasteur and Foundation for Influenza Epidemiology; no conflict of interest.
ASC: Member of the research team of the GIHSN project funded by Sanofi Pasteur and Foundation for Influenza Epidemiology; principal investigator in influenza clinical trials by Activaero and Shionogi; no conflict of interest.
MN: Local coordinator of the I-MOVE+ study funded through the European Union's HORIZON 2020 research and innovation program; no conflict of interest.
AI: Laboratory coordinator of the I-MOVE+ study funded through the European Union's HORIZON 2020 research and innovation program; no conflict of interest.
RB: Member of the I-MOVE+ study funded through the European Union's HORIZON 2020 research and innovation program; no conflict of interest.
DP: Technical project manager for the GIHSN project funded by Sanofi Pasteur and Foundation for Influenza Epidemiology. Principal investigator of the I-MOVE+ study funded through the European Union's HORIZON 2020 research and innovation program; no conflict of interest.
FundingFor patients aged below 65 years old, the study was partially funded by Sanofi Pasteur and Foundation for Influenza Epidemiology, France (GIHSN grant 2016/17), and partially by the National Institute for Infectious Diseases “Prof. Dr. Matei Balş”, Bucharest, Romania.
For patients aged 65 years and older, funding was provided through the study “I-MOVE+ – Hospital-based test negative case control studies to measure seasonal influenza vaccine effectiveness against influenza laboratory confirmed SARI hospitalization among the elderly across the European Union and European Economic Area Member States”. European Union's HORIZON 2020 research and innovation program (grant 6334446). The Society for Infectious Diseases and HIV/AIDS has provided funding to cover the journal's article publishing charge.
NotePartial data from these studies were presented at the 13th Edition of the Scientific Days of the National Institute of Infectious Diseases “Prof. Dr. Matei Balş”, Bucharest, Romania (November 2017), 28th European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain (April 2018), the 36th Annual Meeting of the European Society for Pediatric Infectious Diseases, Malmö, Sweden (May–June 2018) and the 6th Scientific Congress of the Carol Davila University of Medicine and Pharmacy, Bucharest, Romania (June 2018).
Conflicts of interestThe authors declare no conflicts of interest.
The technical platform of the National Institute for Infectious Diseases “Prof. Dr. Matei Balş”, Bucharest, Romania has recently been upgraded through the project “Romanian Center for Applied Bio-Molecular Research in Infectious Diseases”, financed through the Sectorial Operational Program Increasing of Economic Competitiveness (POS CCE) (contract 1871/49153).
We would like to thank the team from Epiconcept, France, and the GIHSN network.
We would like to thank the following colleagues for their contribution in patient enrollment (in alphabetical order): Delia Azamfire, Ioana Baltagi, Emanuel Condria, Ioana Andreea Dărămuş, Aura Dumitrescu, Flavia Flintasu, Elena Ianoşik, Adriana Ana-Maria Iliescu, Lucia Mihaela Papasteri, Alexandra Radu, Georgiana Stan.