Studies about risk factors for mortality in burn children are scarce. We conducted this study to evaluate the risk factors for mortality in pediatric burn patients. We included 110 patients. Mean age was 31.5 months (range: 1 to 204). The burn surface was between 1% and 95%(median 27%) Type of burn was: A or superfitial in 39 patients (36%), AB or intermediate in 19 (17%), and B or full thickness in 52 (47%). Inhalatory injury was present in 52 patients (47%). Invasive procedures were: venous catheter, 90 patients (82%), arterial catheter, 83patients (75.5%), urinary catheter, 86 patients (78%), and mechanical ventilation, 75 patients (68%). In 84 patients, 128 infections were diagnosed. in 53 cases (48%). Multiresistant Pseudomonas aeruginosa and Acynetobacter baumannii were the most common organisms isolated. The median length of hospital stay was 33 days (r: 8-139 days). Seventeen patients (15%) died and 14 of them of infection-related causes. Age≤ <4 years, Garcés 4, full thickness burn, ≥ 40% burn surface, presence of inhalatory syndrome, use of venous catheter, arterial catheter, urinary catheter and mechanical ventilation, positive blood cultures, colistin use in documented multiresistant infections, antifungal use and graft requirement, were identified as risks factors for mortality in the univariate analysis. By multivariate analysis: age ≤4 years, Garcés 4, colistin use in multiresistant infections, mechanical ventilation and graft requirement were independent variables related with mortality.
ConclusionsIn this series of burn children age ≤ 4 years, Garces index score 4, colistin use in documented multiresistant infections, mechanical ventilation and graft requirement were identified as independent variables related with mortality.
Burns are very frequent and affect approximately 1% of the general population every year.1
The immunocompromising effects of burns, hospital stay; diagnostic and therapeutic procedures put these patients at increased risk of morbidity and mortality. In the last few years patients who survived to burn injury has increased, but despite advances in the management of burn patients, infections remain the most common cause of morbidity and mortality following burn injury.1,2
The mortality rate in burn centers varies depending on different factors, like age of patients, being the extremes of life more vulnerable, and particularly the pediatric population aged less than four years. Furthermore, type and mechanisms of burns have also been associated with mortality.
Full thickness burns, burns secondary to flame associated with inhalation injury have higher rate of infections and mortality.1–5
Localization of burns in perineal area and face has increased risk of mortality too.5
In developing countries mortality due to infections is very high and an important risk factor to prevent.
The literature reporting on mortality risk associated with burn in childhood is scarce. Therefore, risk factors for mortality are not well known and strategies for their prevention and the prompt referral of the patients to specialized centers cannot be implemented.
The aim of this study was to evaluate epidemiological features and risk factors for mortality in cases of childhood burn admitted in our tertiary hospital.
Patients and methodsAll children with acute burns admitted to the Burn Center at J. P. Garrahan Hospital between June 2007 and December 2009 were included.
Type of study: prospective and observational study. Patients were followed prospectively during hospitalization and data collection was made through discharge or death.
DefinitionsIndependent variables- (1)
Gender: male–female.
- (2)
Age in months.
- (3)
Type of burn: superficial (A), intermediate (AB), and “full-thickness” or (B).
- (4)
Mechanisms of burns: classified as: flame, scalds, inflammables liquids; explosion, and others mechanisms.
- (5)
Burn surface: Defined as percentage of body according Lund & Bowder chart.5
- (6)
Garcés¿ Index: it is an index of prediction for mortality and is calculated according the following formula6:
40−age of patients+the percentage of burn body surfaces for 1 (burn type A), for 2 (AB) or for 3 (B).
0–60 points: first degree (low risk).
61–90: second degree (moderate risk).
91–120: third degree (severe risk).
≥121: fourth degree (critical).
- (7)
Invasive procedures: use of mechanical ventilation, central venous line, arterial and urinary catheters.
- (8)
Inhalation syndrome: suspected in facial burn, stridor, and/or exposure to heavy smoke and confirmed by endoscopic examination.
- (9)
Type of infections were defined according to The American Burn Association7 and based in clinical and/or microbiological parameters.
- (10)
Positive blood culture.
- (11)
Type of microorganisms isolated in sterile material.
- (12)
Use of colistin: in patients with documented infections by multiple-resistant microorganisms only susceptible to colistin or, in some cases the use was empirical, pending culture results.
- (13)
Use of antifungal drugs.
- (14)
Type of surgical treatment: scarectomy and graft requirement.
Mortality: mortality was considered infection related if the patient had clinical and/or microbiological evidence of infection at the time of death.
MicrobiologyCultures: blood cultures, wound cultures, and urine cultures were taken when appropriate according to clinical features.
Cultures were performed according to the Clinical Laboratory Standard Institute (CLSI) methods. Susceptibility testing was performed according to the CLSI using automated methods.8
Multiresistant Pseudomonas spp. and Acinetobacter spp. were defined as resistant to at least three classes of drugs (e.g. beta-lactam antibiotics, carbapenems, aminoglycosides, and fluoroquinolones).
TreatmentAntibiotic treatment was indicated in the presence of positive cultures and/or according to clinical features.
Statistical analysisData were summarized in frequencies and percentages for categorical variables and as means and ranges (for continuous variables). The Mann–Whitney Rank Sum test was used to assess differences between groups for two continuous variables. Dichotomous variables were analyzed using the Chi-square test (with Yates correction). To estimate the multivariate predictive value of independent covariates for mortality stepwise multiple logistic regression models were used (software in http://statpages.org/logistic.html) including all significant variables in univariate analysis.
The predictive value for each covariant was expressed as the relative risk (RR) and 95% confidence interval. A p-value of ≤0.05 was considered significant for both sides.
This study was approved by the Ethics committee of the Hospital J. P. Garrahan.
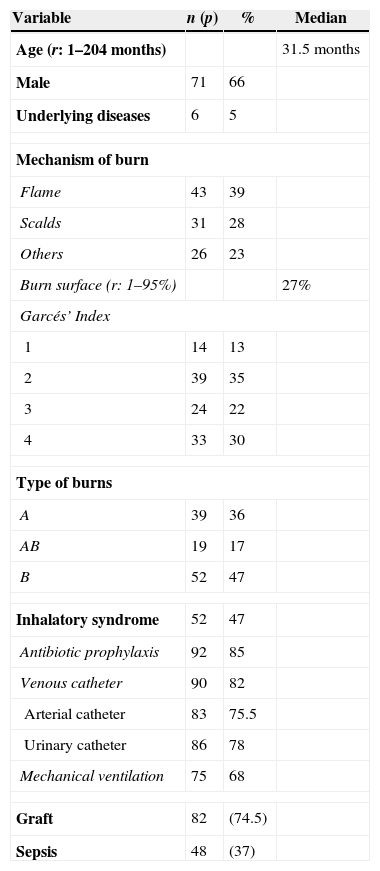
ResultsEpidemiological characteristicsWe included 110 patients.
The median age of patients was 31.5 months (range: 1–204), and 71 patients (65%) were male.
Underlying diseases were found in six patients (5%): genetic syndrome in two patients, one metabolopathy, one oncohematological disease, one cardiopathy, and one patient with neurological disease.
Mechanism of burns was flame in 43 patients (39%); scalds, 31 patients (28%); inflammable liquids, 20 patients (18%); explosion, 8 patients (7%); and other mechanisms, 8 patients (8%).
Clinical and microbiological featuresMedian burn surface affected was 27% (range 1–95%).
Garcés’ index was 1 in 14 patients (13%), 2 in 39 (35%), 3 in 24 (22%) and 4 in 33 (30%).
Type of burn was: A in 39 patients (36%), AB in 19 (17%) and B in 52 (47%).
Localization of burn was variable: head and face in 76 patients (69%), trunk in 54 (49%), upper limb in 57 (52%), lower limbs in 45 (41%), hands in 16 (15%), perineal area in 6 (5.5%), and whole body except perineal area in 10 (9%) patients.
Inhalation syndrome was present in 52 (47%) patients.
Ninety patients (82%) had indwelling venous catheters, 83 (75.5%) patients arterial catheter and 86 (78%) patients urinary catheters.
Seventy-five patients (68%) required mechanical ventilation.
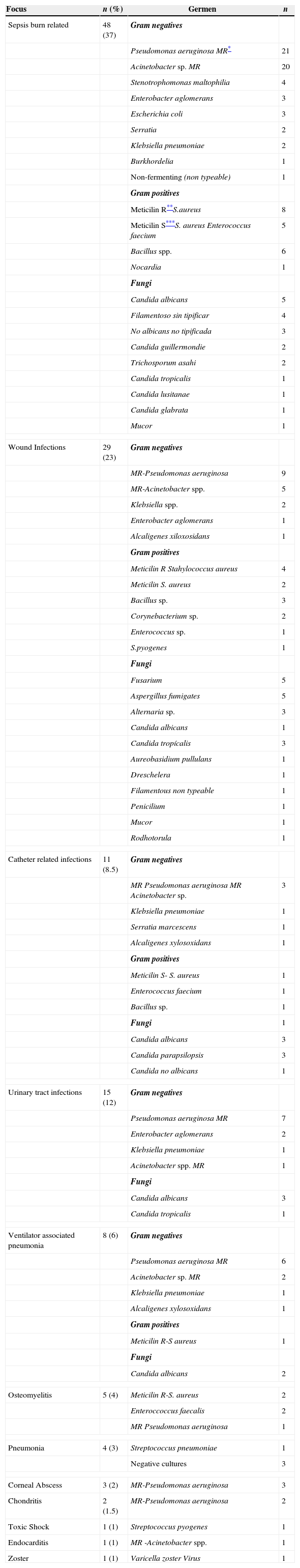
We documented 128 infections in 84 patients being sepsis related to burn injury the most common.
Blood cultures were positive in 49 infections (38%).
Multiresistant Pseudomonas aeruginosa (52 infections) and Acinetobacter spp. (29 infections) were isolated most frequently.
Colistin was used in 68 (62%) patients: empirical use in 28 (41%) and with multiresistant documented infection in 40 (59%) patients.
Antifungal was used in 40 (36%) patients. Scarectomy was required in 90 (82%) patients and graft in 82 (74.5%).
(See Tables 1 and 2.)
Characteristics of the patients (n: 110).
| Variable | n (p) | % | Median |
|---|---|---|---|
| Age (r: 1–204 months) | 31.5 months | ||
| Male | 71 | 66 | |
| Underlying diseases | 6 | 5 | |
| Mechanism of burn | |||
| Flame | 43 | 39 | |
| Scalds | 31 | 28 | |
| Others | 26 | 23 | |
| Burn surface (r: 1–95%) | 27% | ||
| Garcés’ Index | |||
| 1 | 14 | 13 | |
| 2 | 39 | 35 | |
| 3 | 24 | 22 | |
| 4 | 33 | 30 | |
| Type of burns | |||
| A | 39 | 36 | |
| AB | 19 | 17 | |
| B | 52 | 47 | |
| Inhalatory syndrome | 52 | 47 | |
| Antibiotic prophylaxis | 92 | 85 | |
| Venous catheter | 90 | 82 | |
| Arterial catheter | 83 | 75.5 | |
| Urinary catheter | 86 | 78 | |
| Mechanical ventilation | 75 | 68 | |
| Graft | 82 | (74.5) | |
| Sepsis | 48 | (37) | |
Microbiologic features and foci (n: 128 foci in 84 patients).
| Focus | n (%) | Germen | n |
|---|---|---|---|
| Sepsis burn related | 48 (37) | Gram negatives | |
| Pseudomonas aeruginosa MR* | 21 | ||
| Acinetobacter sp. MR | 20 | ||
| Stenotrophomonas maltophilia | 4 | ||
| Enterobacter aglomerans | 3 | ||
| Escherichia coli | 3 | ||
| Serratia | 2 | ||
| Klebsiella pneumoniae | 2 | ||
| Burkhordelia | 1 | ||
| Non-fermenting (non typeable) | 1 | ||
| Gram positives | |||
| Meticilin R**S.aureus | 8 | ||
| Meticilin S***S. aureus Enterococcus faecium | 5 | ||
| Bacillus spp. | 6 | ||
| Nocardia | 1 | ||
| Fungi | |||
| Candida albicans | 5 | ||
| Filamentoso sin tipificar | 4 | ||
| No albicans no tipificada | 3 | ||
| Candida guillermondie | 2 | ||
| Trichosporum asahi | 2 | ||
| Candida tropicalis | 1 | ||
| Candida lusitanae | 1 | ||
| Candida glabrata | 1 | ||
| Mucor | 1 | ||
| Wound Infections | 29 (23) | Gram negatives | |
| MR-Pseudomonas aeruginosa | 9 | ||
| MR-Acinetobacter spp. | 5 | ||
| Klebsiella spp. | 2 | ||
| Enterobacter aglomerans | 1 | ||
| Alcaligenes xiloxosidans | 1 | ||
| Gram positives | |||
| Meticilin R Stahylococcus aureus | 4 | ||
| Meticilin S. aureus | 2 | ||
| Bacillus sp. | 3 | ||
| Corynebacterium sp. | 2 | ||
| Enterococcus sp. | 1 | ||
| S.pyogenes | 1 | ||
| Fungi | |||
| Fusarium | 5 | ||
| Aspergillus fumigates | 5 | ||
| Alternaria sp. | 3 | ||
| Candida albicans | 1 | ||
| Candida tropícalis | 3 | ||
| Aureobasidium pullulans | 1 | ||
| Dreschelera | 1 | ||
| Filamentous non typeable | 1 | ||
| Penicilium | 1 | ||
| Mucor | 1 | ||
| Rodhotorula | 1 | ||
| Catheter related infections | 11 (8.5) | Gram negatives | |
| MR Pseudomonas aeruginosa MR Acinetobacter sp. | 3 | ||
| Klebsiella pneumoniae | 1 | ||
| Serratia marcescens | 1 | ||
| Alcaligenes xylosoxidans | 1 | ||
| Gram positives | |||
| Meticilin S- S. aureus | 1 | ||
| Enterococcus faecium | 1 | ||
| Bacillus sp. | 1 | ||
| Fungi | 1 | ||
| Candida albicans | 3 | ||
| Candida parapsilopsis | 3 | ||
| Candida no albicans | 1 | ||
| Urinary tract infections | 15 (12) | Gram negatives | |
| Pseudomonas aeruginosa MR | 7 | ||
| Enterobacter aglomerans | 2 | ||
| Klebsiella pneumoniae | 1 | ||
| Acinetobacter spp. MR | 1 | ||
| Fungi | |||
| Candida albicans | 3 | ||
| Candida tropicalis | 1 | ||
| Ventilator associated pneumonia | 8 (6) | Gram negatives | |
| Pseudomonas aeruginosa MR | 6 | ||
| Acinetobacter sp. MR | 2 | ||
| Klebsiella pneumoniae | 1 | ||
| Alcaligenes xylosoxidans | 1 | ||
| Gram positives | |||
| Meticilin R-S aureus | 1 | ||
| Fungi | |||
| Candida albicans | 2 | ||
| Osteomyelitis | 5 (4) | Meticilin R-S. aureus | 2 |
| Enteroccoccus faecalis | 2 | ||
| MR Pseudomonas aeruginosa | 1 | ||
| Pneumonia | 4 (3) | Streptococcus pneumoniae | 1 |
| Negative cultures | 3 | ||
| Corneal Abscess | 3 (2) | MR-Pseudomonas aeruginosa | 3 |
| Chondritis | 2 (1.5) | MR-Pseudomonas aeruginosa | 2 |
| Toxic Shock | 1 (1) | Streptococcus pyogenes | 1 |
| Endocarditis | 1 (1) | MR -Acinetobacter spp. | 1 |
| Zoster | 1 (1) | Varicella zoster Virus | 1 |
Median length of stay was 37 days (range 1–139 days).
Seventeen patients (15%) died, 14 (82%) for infectious related causes.
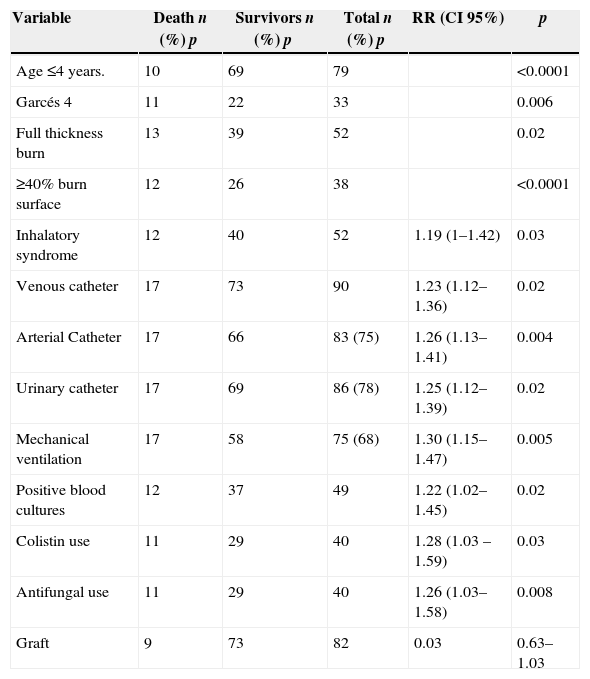
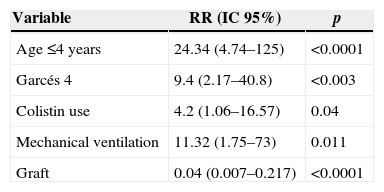
Independent risk factors for mortality were: age ≤4 years, Garcés 4, full thickness burn, ≥40% burn surface, inhalation syndrome, venous catheter, arterial catheter, urinary catheter, mechanical ventilation, positive blood cultures, colistin use in documented multiresistant infections, antifungal use, and graft requirement. By multivariate analysis: age ≤4 years, Garcés 4, colistin use in documented multiresistant infections, and mechanical ventilation were independent variables related with mortality and graft requirement was a protective factor for mortality. A comparison between survivors and non-survivors by univariate and multivariate analysis is shown in Tables 3 and 4.
Risk factors for mortality: bivariate analysis (n: 110, p).
| Variable | Death n (%) p | Survivors n (%) p | Total n (%) p | RR (CI 95%) | p |
|---|---|---|---|---|---|
| Age ≤4 years. | 10 | 69 | 79 | <0.0001 | |
| Garcés 4 | 11 | 22 | 33 | 0.006 | |
| Full thickness burn | 13 | 39 | 52 | 0.02 | |
| ≥40% burn surface | 12 | 26 | 38 | <0.0001 | |
| Inhalatory syndrome | 12 | 40 | 52 | 1.19 (1–1.42) | 0.03 |
| Venous catheter | 17 | 73 | 90 | 1.23 (1.12–1.36) | 0.02 |
| Arterial Catheter | 17 | 66 | 83 (75) | 1.26 (1.13–1.41) | 0.004 |
| Urinary catheter | 17 | 69 | 86 (78) | 1.25 (1.12–1.39) | 0.02 |
| Mechanical ventilation | 17 | 58 | 75 (68) | 1.30 (1.15–1.47) | 0.005 |
| Positive blood cultures | 12 | 37 | 49 | 1.22 (1.02–1.45) | 0.02 |
| Colistin use | 11 | 29 | 40 | 1.28 (1.03 –1.59) | 0.03 |
| Antifungal use | 11 | 29 | 40 | 1.26 (1.03–1.58) | 0.008 |
| Graft | 9 | 73 | 82 | 0.03 | 0.63–1.03 |
Mortality in burn patients is an important complication and studies about risk factors in the pediatric population remained poorly delineated and scarce.1–5
Overall mortality rates in burn patients are variable ranging between 3.5% and 12%, depending on the extension of the burn, age of the patient and presence of multiple organ failure.1
Fungal infection is an important cause of morbidity and mortality in burn patients.1
There are a few reports in pediatrics burn patients.
A burn patient may die because of different causes: shock in the first hours post-burn, respiratory failure, usually secondary to the inhalation syndrome; multiple organ failure secondary to the extension and deepness of the burn wound, among others.
Nevertheless, in burn units infections and more specifically sepsis are the most common cause of death.2,3
Pediatric burn patients are susceptible to a broad spectrum of infections which represent the most common and severe complication in this population.9–16
Invasive procedures, misuse of antibiotics among others are key factors that in association with a susceptible host due to impaired immune system and loss of skin as the first defense barriers facilitate infectious complications that may lead to death.
Awareness of risk factors related to increased mortality in burn patients allow for extreme safety measures and adequate referral of patients to tertiary care centers to be implemented.2,5
Gender was not associated to mortality in our series, unlike the findings by O¿Keefe et al.17 who reported increased mortality in adult women.
Age less than four years has been considered as a major risk factor for mortality in children3,5,18 in line with our report.
A high Garcés’ score, particularly 4, was significantly associated with a higher risk of death; however, when this index was included in multivariate models with other parameters, such as age, deepness and burn surfaces it was longer independently associated with mortality.
Type B, “full thickness” has been viewed as a risk factor by several authors,5,9 a finding that was confirmed in the present study.
Inhalation syndrome is a clear and strong predictor of mortality.3,5,9 In univariate analysis inhalation syndrome was a predictor of mortality in our patients but it significance in multivariate analysis. Nevertheless, requirement of mechanical ventilation was significantly related with mortality and may indirectly reflect this condition in some cases.
Mechanical ventilation is clearly related with mortality in other studies.1–3
Majority of burn patients required invasive procedures such as indwelling catheters frequently used in intensive care units for resuscitation and fluids and antibiotics administration and/or urinary catheters, which are also associated with increased risk of infections and mortality.14 This finding was confirmed by univariate analysis in our series.
Blood stream infection in burn patients has been previously associated with mortality3 as it did in our series by univariate analysis.
Infections complications are related with increased risk of mortality and the development of sepsis is a predictor of poor outcome.2,3 Fourteen patients in our study died because of sepsis. Multiresistant Acinetobacter spp. and P. aeruginosa have emerged as important microorganisms in critical areas like burn units and are related with increased mortality in these areas.19–21
A high proportion of infections in our study were caused by Multiresistant P. aeruginosa and Acinetobacter spp.
Colistin, a bactericidal antibiotic, polymyxin derived, was used in the 1960s for the treatment of infections by Gram-negative bacteria.
Because of its side effects and the development of safer drugs, its use was abandoned in the 1980s. The emergence of multidrug resistant (MDR) Gram-negative bacteria and the lack of new antibiotics to combat them, have led to the revival of polymyxins.21–23
The use of Colistin in documented multiresistant infections was associated to mortality in our series. It is possible that this association indirectly reflects the impact of multiresistant Gram-negative bacteria, although univariate and multivariate analyses did not show a relationship between type of infections, type of microorganisms and mortality. However, presence of non-catheter related positive blood cultures, and the use of colistin in documented multiresitant microorganisms should indicate an indirect correlation between this variables and death.
Fungal infection, not colonization, has been recognized as an important cause of morbidity and mortality in burn patients.1–26 However, fungal infection appears in more severely injured patients with the high percentage of burned body surface, and if the wounds remained opened for prolonged periods, the use of antibiotics and long hospital stay could be related with the propensity to acquire fungal infections.24 The use of antifungal drugs was a predictor of poor outcome in our patients by univariate analysis, but not in multivariate analysis. Early surgical excision and temporary or permanent closure with grafts are important measures for prevention of infections and related deaths.3,26 In our series graft requirement was a protective factor for mortality as pointed out in the literature but scarectomy was not an independent variable related to mortality.
In the study by Dermijian5 to evaluate risk factors for mortality, burn surface greater than 40%, presence of inhalation syndrome, location glutea or in perineal area, and inadequate referral of patients were independent risk factors for mortality. In our series burn surface was a predictor for mortality but the location and inhalation syndrome were not independently associated with mortality in multivariate analysis. We could not evaluate type of referral.
Mortality rates reported in literature are lower than the rate found in our study,1–27 but a limitation of the present report is that the data were collected in a national tertiary referral center which may have caused patient selection bias, including a higher proportion of critically ill e patients.
Although the sample size in our study was not large enough to draw definitive conclusions for some of the studied variables, the findings are important due to the paucity of systematic studies about this topic in children. Further research is necessary to support the data.
Knowledge of risk factors for mortality could be useful for the development of strategies to prevent this outcome which together with adequate referral to specialized centers will warrant a better management of these vulnerable patients.
ConclusionAge ≤4 years, Garcés’ score 4, colistin use in documented multiresistant infections, mechanical ventilation and graft requirement were independent variables associated with mortality.
Conflicts of interestThe authors declare no conflicts of interest.