Healthcare Associated Infections constitute an important problem in Neonatal Units and invasive devices are frequently involved. However, studies on risk factors of newborns who undergo surgical procedures are scarce.
ObjectiveTo identify risk factors for laboratory-confirmed bloodstream infection in neonates undergoing surgical procedures.
MethodsThis case–control study was conducted from January 2008 to May 2011, in a referral center. Cases were of 21 newborns who underwent surgery and presented the first episode of laboratory-confirmed bloodstream infection. Control was 42 newborns who underwent surgical procedures without notification of laboratory-confirmed bloodstream infection in the study period. Information was obtained from the database of the Hospital Infection Control Committee Notification of infections and related clinical data of patients that routinely collected by trained professionals and follow the recommendations of Agência Nacional de Vigilância Sanitária and analyzed with Statistical Package for Social Sciences.
ResultsDuring the study period, 1141 patients were admitted to Neonatal Unit and 582 Healthcare Associated Infections were reported (incidence-density of 25.75 Healthcare Associated Infections/patient-days). In the comparative analysis, a higher proportion of laboratory-confirmed bloodstream infection was observed in preterm infants undergoing surgery (p=0.03) and use of non-invasive ventilation was a protective factor (p=0.048). Statistically significant difference was also observed for mechanical ventilation duration (p=0.004), duration of non-invasive ventilation (p=0.04), and parenteral nutrition duration (p=0.003). In multivariate analysis duration of parenteral nutrition remained significantly associated with laboratory-confirmed bloodstream infection (p=0.041).
ConclusionsShortening time on parenteral nutrition whenever possible and preference for non-invasive ventilation in neonates undergoing surgery should be considered in the assistance of these patients, with the goal of reducing Healthcare Associated Infections, especially laboratory-confirmed bloodstream infection.
Healthcare Associated Infections (HAI) constitute an important problem in neonatal units and are associated to higher morbidity, mortality and duration of hospitalization of newborns.1–3
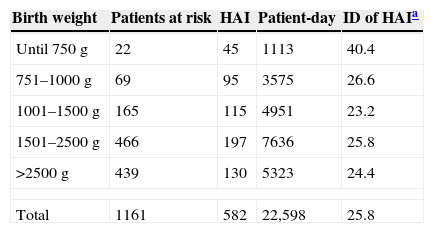
Rates of infection reported in international studies range from 0.1% in newborns at term to 20% in premature infants, and are inversely related to birth weight.4,5 In Brazil, higher rates are observed – they can reach 69.3% – and infection affects mainly infants weighing less than 1000g.6–8 The incidence density (ID) also varies with birth weight, from approximately 12% per 1000 patient-days in newborns weighing more than 2500g, to 51.9% per 1000 patient-days in those with less than 1000g.7–9
There are several risk factors associated with nosocomial infections in neonatal units, such as prematurity, mechanical ventilation (MV) and central venous catheter (CVC), length of stay in the unit, as well as parenteral nutrition (PN), antibiotics, and malformations.2,6,10,11 Surgical procedures determine surveillance of wound infection and surveillance recommendations to investigate this event.12–14 But risk factors for laboratory-confirmed bloodstream infection (LCBI) in surgical neonates are scarce nationally and internationally. In a previous study at the same Neonatal Unit for Progressive Care (NUPC) where this study was conducted, surgery, MV, and CVC were identified as independent risk factors for LCBI.15
The aim of this study was to identify risk factors for LCBI in neonates undergoing surgical procedures at a NUPC in a referral hospital.
Patients and methodsThe NUPC at Hospital das Clínicas of Universidade Federal de Minas Gerais is a tertiary referral center for assistance of patients with diverse clinical conditions, including high-risk newborns. This case–control study was conducted from January 2008 to May 2011.
Case definitionNewborns (NB) who underwent surgery and presented the first episode of LCBI, defined by the reporting criteria of infection in infants under one year – according to the National Agency for Sanitary Surveillance (Agência Nacional de Vigilância Sanitária – ANVISA),12 – after the procedure, were the subject of this study. Only the first episode of LCBI was considered; therefore, patients were included only once.
Criteria for notification of infection attributed to commensal microorganisms (e.g. coagulase-negative Staphylococcus) were not included, due to the necessity of isolation in two samples of blood culture associated with clinical signs.12,13
Control definitionControls were newborns who underwent surgical procedures without any signs or symptoms of sepsis or any notification of LCBI in the post-operative period. Two controls per case matched for weight were selected.
Data collectionThe information was obtained from the database of the Hospital Infection Control Committee (HICC) and patient records. Variables included were: type of surgery, birth weight divided by age, prematurity (gestational age less than 37 weeks), presence of malformations, use of invasive devices (CVC and MV), use of non-invasive ventilation (NIV), use of continuous positive airway pressure (CPAP), chest drain, use of PN, and prior antibiotic use (ATM). In addition, surgical fasting and fasting duration, mortality, time (in days) with CVC, MV, NIV in CPAP, chest tube, PN, ATM and number of ATM regimens were also evaluated.
Statistical analysisThe software used for analysis was the Statistical Package for Social Sciences (SPSS), version 13.0. The distribution of patients by birth weight (used for matching) and type of surgery was considered only in the descriptive analysis. For the comparative analysis between groups (case vs. control), χ2 test was used for categorical variables. For quantitative variables, Student's t test or Mann–Whitney test was used, according to analysis of variance test evaluated by Levine. Statistical significance was considered at 5%. Multivariate analysis was performed by binary logistic regression, considering the dependent variable the occurrence of LCBI, and as predictors the risk factors significantly (p<0.05) associated with LCBI in univariate analysis. Individual contribution of each risk factor was tested (Wald chi-square), thereby eliminating the overlap between the predictors. This study is part of the activities of Surveillance and Control of Infection in Neonatology, and it was approved by the Institutional Review Board (ETIC 312/08).
ResultsDuring the study period, 1141 patients were admitted to NUPC, with 22,598 patient-days. A total of 582 HAI were reported with an ID of 25.75/1000 patient-days. From these infections, 126 were LCBI with ID HAI of 5.58/1000 patient-days (Table 1).
Distribution of newborns according to weight range and incidence density of healthcare associated infections (HAI), Neonatal Unit for Progressive Care, HC/UFMG, January 2008 to April 2011.
| Birth weight | Patients at risk | HAI | Patient-day | ID of HAIa |
|---|---|---|---|---|
| Until 750g | 22 | 45 | 1113 | 40.4 |
| 751–1000g | 69 | 95 | 3575 | 26.6 |
| 1001–1500g | 165 | 115 | 4951 | 23.2 |
| 1501–2500g | 466 | 197 | 7636 | 25.8 |
| >2500g | 439 | 130 | 5323 | 24.4 |
| Total | 1161 | 582 | 22,598 | 25.8 |
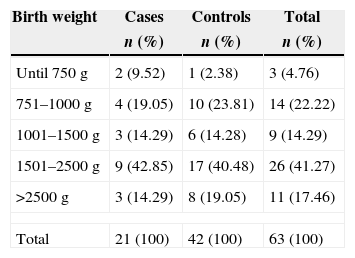
Among high-risk patients admitted in the period, we identified 21 cases of surgical patients with notification of LCBI, who were matched to 42 neonates undergoing surgical procedures, but without LCBI. Birth weight distribution of cases and controls is shown in Table 2, with higher frequency of patients in the weight range 1501–2500g (41.27%), followed by patients weighing between 751 and 1000g (22.22%).
Birth weight distribution of newborns who underwent surgical procedures, Neonatal Unit for Progressive Care, HC/UFMG, January 2008–April 2011.
| Birth weight | Cases | Controls | Total |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Until 750g | 2 (9.52) | 1 (2.38) | 3 (4.76) |
| 751–1000g | 4 (19.05) | 10 (23.81) | 14 (22.22) |
| 1001–1500g | 3 (14.29) | 6 (14.28) | 9 (14.29) |
| 1501–2500g | 9 (42.85) | 17 (40.48) | 26 (41.27) |
| >2500g | 3 (14.29) | 8 (19.05) | 11 (17.46) |
| Total | 21 (100) | 42 (100) | 63 (100) |
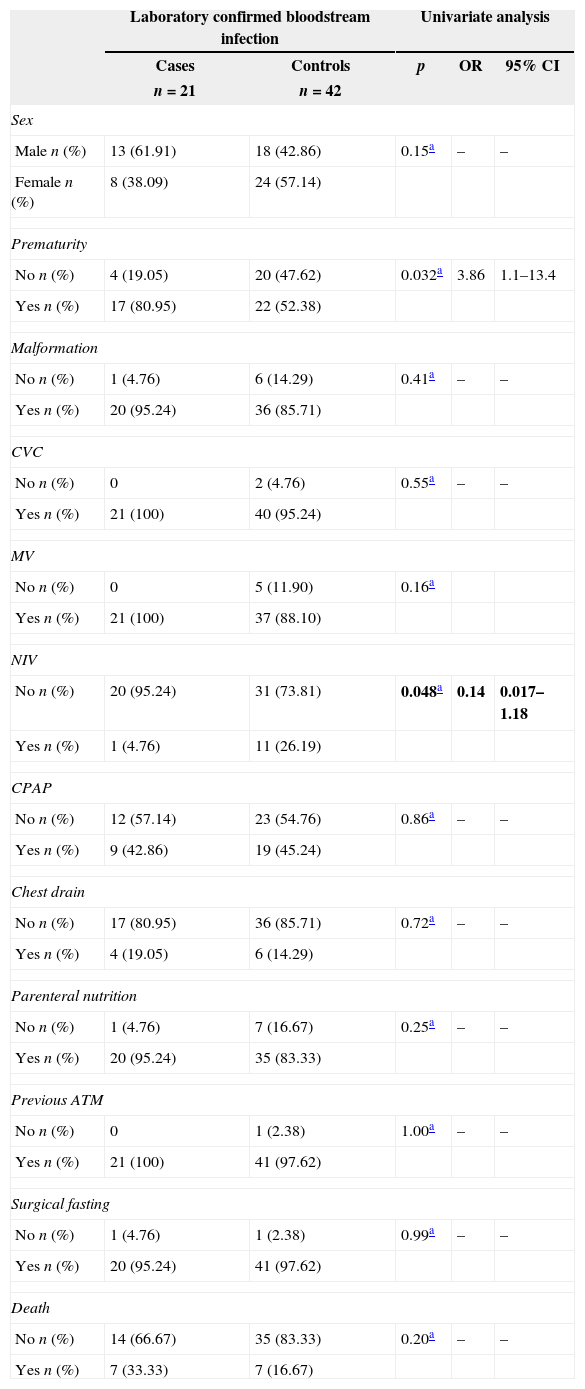
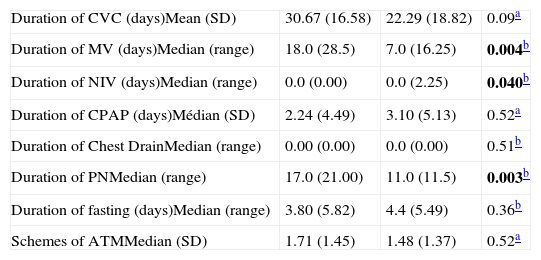
Preterm infants undergoing surgery had 3.75 greater chance of LCBI than term babies. Although statistical analisis presented p=0,048 for NIV, it was not significant considering that 95% CI included 1 (Table 3). For continuous variables, statistically significant difference was observed for MV duration (greater in cases), duration of NIV (greater in controls), and PN duration (greater in cases) (Table 4).
Association of risk factors with laboratory confirmed bloodstream infection in neonates undergoing surgery. Neonatal Unit for Progressive Care. HC/UFMG. January 2008–April 2011.
| Laboratory confirmed bloodstream infection | Univariate analysis | ||||
|---|---|---|---|---|---|
| Cases | Controls | p | OR | 95% CI | |
| n=21 | n=42 | ||||
| Sex | |||||
| Male n (%) | 13 (61.91) | 18 (42.86) | 0.15a | – | – |
| Female n (%) | 8 (38.09) | 24 (57.14) | |||
| Prematurity | |||||
| No n (%) | 4 (19.05) | 20 (47.62) | 0.032a | 3.86 | 1.1–13.4 |
| Yes n (%) | 17 (80.95) | 22 (52.38) | |||
| Malformation | |||||
| No n (%) | 1 (4.76) | 6 (14.29) | 0.41a | – | – |
| Yes n (%) | 20 (95.24) | 36 (85.71) | |||
| CVC | |||||
| No n (%) | 0 | 2 (4.76) | 0.55a | – | – |
| Yes n (%) | 21 (100) | 40 (95.24) | |||
| MV | |||||
| No n (%) | 0 | 5 (11.90) | 0.16a | ||
| Yes n (%) | 21 (100) | 37 (88.10) | |||
| NIV | |||||
| No n (%) | 20 (95.24) | 31 (73.81) | 0.048a | 0.14 | 0.017–1.18 |
| Yes n (%) | 1 (4.76) | 11 (26.19) | |||
| CPAP | |||||
| No n (%) | 12 (57.14) | 23 (54.76) | 0.86a | – | – |
| Yes n (%) | 9 (42.86) | 19 (45.24) | |||
| Chest drain | |||||
| No n (%) | 17 (80.95) | 36 (85.71) | 0.72a | – | – |
| Yes n (%) | 4 (19.05) | 6 (14.29) | |||
| Parenteral nutrition | |||||
| No n (%) | 1 (4.76) | 7 (16.67) | 0.25a | – | – |
| Yes n (%) | 20 (95.24) | 35 (83.33) | |||
| Previous ATM | |||||
| No n (%) | 0 | 1 (2.38) | 1.00a | – | – |
| Yes n (%) | 21 (100) | 41 (97.62) | |||
| Surgical fasting | |||||
| No n (%) | 1 (4.76) | 1 (2.38) | 0.99a | – | – |
| Yes n (%) | 20 (95.24) | 41 (97.62) | |||
| Death | |||||
| No n (%) | 14 (66.67) | 35 (83.33) | 0.20a | – | – |
| Yes n (%) | 7 (33.33) | 7 (16.67) | |||
OR, odds ratio; CI, confidence interval; CVC, central venous catheter; MV, mechanical ventilation; NIV, non invasive ventilation; CPAP, continuous positive airway pressure; ATM, antimicrobials; SD, standard deviation.
Continuous variables associated with laboratory confirmed bloodstream infection in neonates undergoing surgery. Neonatal Unit for Progressive Care. HC/UFMG. January 2008–April 2011.
| Duration of CVC (days)Mean (SD) | 30.67 (16.58) | 22.29 (18.82) | 0.09a |
| Duration of MV (days)Median (range) | 18.0 (28.5) | 7.0 (16.25) | 0.004b |
| Duration of NIV (days)Median (range) | 0.0 (0.00) | 0.0 (2.25) | 0.040b |
| Duration of CPAP (days)Médian (SD) | 2.24 (4.49) | 3.10 (5.13) | 0.52a |
| Duration of Chest DrainMedian (range) | 0.00 (0.00) | 0.0 (0.00) | 0.51b |
| Duration of PNMedian (range) | 17.0 (21.00) | 11.0 (11.5) | 0.003b |
| Duration of fasting (days)Median (range) | 3.80 (5.82) | 4.4 (5.49) | 0.36b |
| Schemes of ATMMedian (SD) | 1.71 (1.45) | 1.48 (1.37) | 0.52a |
CVC, central venous catheter; MV, mechanical ventilation; NIV, non invasive ventilation; CPAP, continuous positive airway pressure; ATM, antimicrobials; SD, standard deviation.
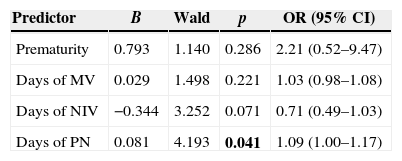
Variables included in the multivariate logistic regression model were prematurity (yes or no), duration of MV, NIV, and PN (number of days). The model increased the predictive value of 66.7–84.1%, correctly identifying 13 of the 21 cases of LCBI (61.9%) and 40 of 42 controls (95.2%). Length of PN was the only risk factor that remained independently associated with LCBI (p=0.041). Each day on PN increased by 9% (95% CI OR: 1.00–1.17) the probability of LCBI. Although not statistically significant, duration of NIV tended to be a protective factor (p=0.071), as each day on NIV was associated with a decrease in the probability of LCBI by 29% (95% CI OR: 0.49–1.03). Prematurity and time on MV were not independently associated with LCBI in the final model (p=0.29 and p=0.22, respectively) (Table 5).
Logistic regression model for predicting laboratory-confirmed bloodstream infection in neonates undergoing surgical procedures. Neonatal Unit for Progressive Care. HC/UFMG. January 2008–April 2011.
| Predictor | B | Wald | p | OR (95% CI) |
|---|---|---|---|---|
| Prematurity | 0.793 | 1.140 | 0.286 | 2.21 (0.52–9.47) |
| Days of MV | 0.029 | 1.498 | 0.221 | 1.03 (0.98–1.08) |
| Days of NIV | −0.344 | 3.252 | 0.071 | 0.71 (0.49–1.03) |
| Days of PN | 0.081 | 4.193 | 0.041 | 1.09 (1.00–1.17) |
B, slope from logistic regression equation; Wald, Wald chi-square; OR, odds ratio predicted by the model; CI, confidence interval; MV, mechanical ventilation; NIV, non-invasive ventilation; PN, parenteral nutrition.
In neonates undergoing surgery, prematurity, duration of MV and PN use were identified as risk factors for progression to LCBI, but only duration of PN use has remained independently associated (p=0.041) in the multivariate analysis. Many reports in international10,16–18 and national2,6 literature define these variables as predictors of sepsis. However, none of the studies were designed to assess risk factors for LCBI in newborns who underwent surgical procedures.
In a previous study15 surgical procedure itself was found to be an independent risk factor for LCBI in newborns. Mokaddas et al.19 evaluated patients with suspected sepsis in a Pediatric Surgical Unit and 2.3% of them had LCBI. Of all surgical pediatric patients, 82% had congenital abnormalities and 87% required surgical interventions. Bhattacharyya et al.20 identified a 6.2% rate of infection reported among surgical patients evaluated, although the authors have not included infections other than LCBI. Higher prevalence of infection in these children was associated to nutritional status (p<0.0001) and several surgical interventions (p<0.0001). Besides, the studies were conducted in the Pediatric Intensive Care Unit and was not restricted to newborns. Nonetheless, neonates were at increased risk of sepsis when compared to other children (4.2% <0.05).
It should be considered that patients who undergo surgical procedures are vulnerable to complications (such as extubation failure and diet intolerance) and they need prolonged PN, as identified in the present study as risk factor for LCBI. They also have longer length of stay in the Neonatal Intensive Care Unit with frequent use invasive devices, such as MV, associated with LCBI in univariate analysis.
Zakariya et al.21 studied only infants who developed LCBI and also identified MV as a risk factor in the logistic regression model with an OR=3.59 (CI 95%=1.16–11.07). However, the authors included all neonates, not only those undergoing surgery.
A study by Su et al.18 included only neonates and identified a higher proportion of nosocomial infection in patients undergoing surgical procedures (13% compared to 6.9%; p<0001). In multivariate analysis, MV and PN remained independently associated with infection, increasing the risk by 21% and 30%, respectively.
Another study in the same city conducted in a private Neonatal Intensive Care Unit,2 which included not only newborns undergoing surgery, also demonstrated that invasive devices were associated with HAI in this population. In multivariate analysis, the use and length of MV was also associated with BSI not associated with CVC. Other risk factors for sepsis in newborns described in the literature – such as prematurity, congenital anomalies, length of antibiotic therapy, and use of CVC2,10,17,18 did not turn out to be associated with LCBI in this study, although prematurity was significantly associated in univariate analysis and preterm infants presented 3.75 greater chance of LCBI when undergoing surgery. It is known that prematurity is directly related to higher rates of sepsis, but most studies focus on birth weight, which is inversely associated to infection.1,2,10,18 LCBI rates of 50% were observed in newborns under 750g,4 as well as ID of 37 per 1000 patient-days.15
Stoll et al.22 studied extreme preterm infants according to gestational age and also found high mortality in these patients, in addition to rates of infection of 33–38%. Auriti et al.23 also identified different risk factors for newborns classified as very low birth weight (VLBW), considering cumulative probability of having infection more prevalent in this group. Risk factors included gestational age<28 weeks and CPAP use. Invasive procedures were associated with higher risk as their duration increased, with the highest risk associated with MV for seven days or more (RR: 3.82; 95% CI 2.83–5.17). These authors also reported as risk factors for infection the use of PN (OR: 8.1; 95% CI 3.2–20.5) and malformations (OR: 2.1; 95% CI 1.5–3.5), especially for heavier newborns, which is confounded by the need for surgical procedures.
In the present study infection was not associated with CPAP. However, the use of NIV might have been a protective factor for LCBI (1 case compared to 11 controls), reducing the chance of LCBI by 86%. Although OR was 0.14, the 95% CI ranged from 0.017 to 1.18. Other studies have shown reduced work of breathing in newborns undergoing NIV, compared to those who used Continuous Positive Pressure Airway, without presenting complications, such as necrotizing enterocolitis and gastrointestinal perforations. However, it was not considered for risk factor analysis.24–26 It should be pointed out that NVI is a less invasive ventilatory support, thus reducing several risks of MV.
There are significant variations in predictors of HAI among neonatal Intensive Care Units, predominating sepsis. Rates of infection in newborns vary between 4.7%16 and 69.3%.1–10,21,27–30 Despite most of the referred studies having not used index by patient-days, their results are in line with the overall density of infections found in this study – 25.75 per 1000 patient-days. However, when considering only LCBI, the ID was 5.58/1000 patient-days, which may raise the issue of notification for clinically suspected sepsis, which is no longer recommended by the NHSN criteria.13
The limitation of this study was the small number of patients and the difficulty of matching newborns who did not undergo surgical procedures: hospitalization and weight range were not considered, only patients undergoing surgery without LCBI during the study period. However, there are few studies in the literature that were carried on in neonatal units with patients undergoing surgical procedures and they did not include only patients with LCBI as case.
Due to the lack of specific signs and symptoms of neonatal sepsis, so that other diagnoses could be ruled out, we have chosen to include only neonates with LCBI, considering that adequate blood cultures are not always obtained, owing to technical difficulties of collecting samples in infants. The exclusion of patients with sepsis caused by commensal microorganisms can create a selection bias, underestimating the number of cases, since most studies6–9 report coagulase negative Staphylococcus as the main agent of neonatal sepsis. However, the goal of this study was to increase the specificity of diagnosis and avoid inclusion of cases diagnosed by only one blood culture of these agents.
When possible, shortening the time on parenteral nutrition with progression of enteral diet and preference for noninvasive ventilation in neonates undergoing surgery admitted to the Intensive Care Unit should be considered in the care of these patients, with the goal of reducing HAI, especially LCBI.
Conflicts of interestThe authors declare no conflicts of interest.