During the second half of the twentieth century, neurologic sequelae associated with central nervous system impairment caused by Rickettsia rickettsii were studied widely and exclusively in the United States. We present the case of a Mexican pediatric patient with neurologic sequelae 10 years after an acute infection by R. rickettsii.
Rocky mountain spotted fever (RMSF), caused by Rickettsia rickettsii, is a highly lethal tick-borne infectious disease. Its distribution is limited to the Americas, where different tick species such as Dermacentor, Rhipicephalus and Amblyomma are recognized as competent vectors.1
In Mexico, cases of RMSF have been described since 1940; the Rhipicephalus sanguineus tick being the most responsible for its transmission. After multiple decades of inactivity, the disease again emerged in the northern Mexican states at the beginning of the twentieth century, and cases in Yucatan in the last decade.2–4
Although the severity of the infection is well recognized, there are regional variations of lethality that fluctuate from 5 to 10% in the United States, to approximately 30% in Mexico. Inappropriate medical management of the cases, differential diagnosis between other less lethal rickettsioses, and differences in virulence of the R. rickettsii strains would explain these variations.1,2,5
Symptoms may include fever, headache, photophobia, general discomfort, myalgias, and petechial eruption that begins in the wrists and ankles and extends to the trunk. The rash may not be present in <15% of patients and the classic triad of fever, headache and rash is quite suggestive of the disease. Severe cases include meningoencephalitis, acute renal failure, acute respiratory distress syndrome, cutaneous necrosis, shock, and arrhythmias.6 Severe neurologic signs of RMSF include focal neurologic deficits as upper or lower motor neuron lesions, hearing loss, neuragenic bladder, delirium, coma and generalized tonic–clonic seizures.7
The characteristics and neurologic sequelae caused by RMSF were widely studied in the second half of the twentieth century in the United States. Although the illness is also endemic in other countries of the Americas such as Mexico, Costa Rica, Panama, Colombia, Brazil, and Argentina, neurologic involvement have not been documented in these countries. This study presents the case of a Mexican female with persistent language and psychomotor neurologic sequelae 10 years after Rickettsia rickettsii acute infection.
Case reportIn 2007, a Mayan 9-year-old female from the indigenous community of Tahdziú, Yucatan, Mexico, living in highly unsanitary conditions and constantly in contact with tick-infected undomesticated animals, seeks medical attention at the local health center of her community with fever for one day, receiving antipyretic treatment at that time. Over the following three days, she additionally experienced asthenia, adynamia, headache, abdominal colic, nausea, vomiting, anorexia, polyarthritis and polymyositis. On the sixth day after febrile onset, she began with photophobia, dysphagia, obtundation; and later, generalized tonic–clonic seizures, for which she was transferred to the General Hospital Agostín O’Horan in Merida. Here she was admitted to the pediatric service with probable diagnosis of neuroinfection.
Upon admission to the hospital, the patient was found lethargic with a Glasgow coma scale of 8, showing vestibular signs, nucal rigidity positive Brudzinski and Kernig signs, hyperesthesia, ataxia, persistent fever above 39°C (102.2°F), anasarca, pulmonary edema, hepatomegaly, and maculopapular rash on the face and extremities.
Initial laboratory studies revealed leukocytosis, transaminitis and coagulopathy. Lumbar puncture showed no signs of neuroinfection (abundant red erythrocytes, decreased leukocytes, proteins at 137mg/dL). Serology for Dengue, Leptospira, and West Nile Virus were negative. On the tenth day of febrile illness, IgM anti-Rickettsia rickettsii Immunofluorescence Assay (IFA) was performed and turned out positive with a titer of 1:1024, as well as amplification and sequencing of the rickettsial gene fragment 17kDa through blood sample, resulting 98% homologous to Rickettsia rickettsii.
Given general worsening of the patient's status, she was transferred to the Pediatric Intensive Care Unit, where she stayed for five days in critical condition, during which time intravenous chloramphenicol was initiated with dosing at 50mg/kg/day, given every four hours for five days.
Twenty-three days later she is discharged from the hospital with neuromotor deficits including distal upper and lower extremity spasticity, as well as motor aphasia. With poor functional prognosis, she is prescribed Phenytoin as anticonvulsant therapy, Citicoline for neuroprotection, physical therapy, and a referral to neurology for follow-up.
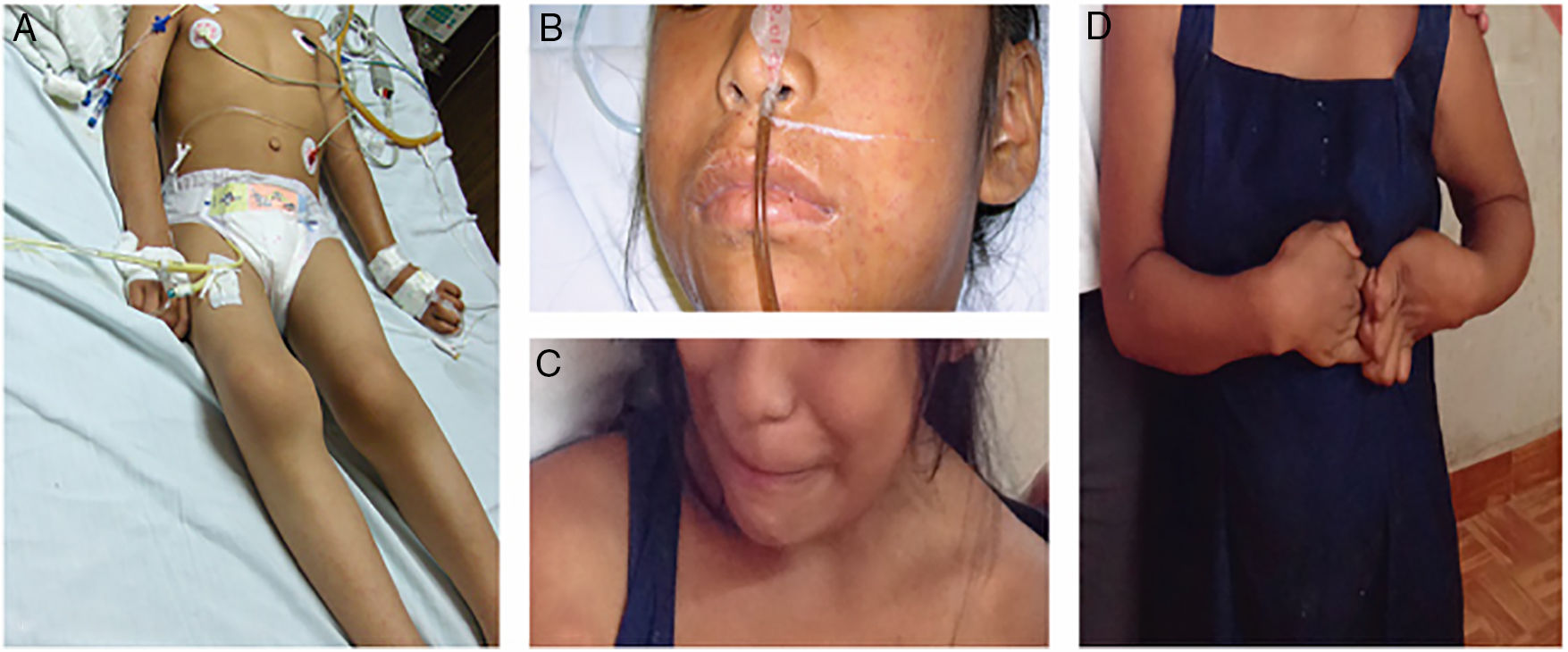
In 2017, 10 years after her initial clinical presentation, a home visit is made by the team of researchers accompanied by a physician. The patient, now aged 19, is found conscious, reactive, cooperative, with sardonic facies, sialorrhea, global aphasia, ataxic gait, lower extremity muscular atrophy, and upper extremity spasticity at grade 3 on Ashworth Scale (Fig. 1).
Patient with neurologic sequelae from Rickettsia rickettsii. (A) 9-Year-old pediatric patient in critical condition during initial illness (2007). (B) Maculopapular rash with facial edema (2007). (C) 19-Year-old patient with sardonic facies (2017). (D) Rigidity and spasticity in upper extremities (2017).
The mother states that the patient did not have appropriate neurologic follow up nor physical therapy due to limited resources as well as long travel required to the hospital where she was receiving care. She had not had another neurologic event since her initial hospitalization. The neurologic sequelae of the patient limit her ability to participate and be socially accepted.
DiscussionThe clinical presentation in this patient are similar in type and frequency to those described in the literature.1,2,6 The neurological complications occur in less than 40% of the patients and include altered mental state, meningism, convulsions, neuralgias, confusion, photophobia, and psychosis. In the present case, all of these were present except psychosis.
It is worth mentioning that the neurologic manifestations are most frequently observed in patients with prolonged hospital course.8 This patient was hospitalized for more than 20 days, five of which were in the Pediatric Intensive Care Unit.
The patient presented with multiple clinical and epidemiological signs warranting high suspicion of the diagnosis of RMSF. Nonetheless, this was not established until the tenth day of illness, which caused delay in treatment, and as consequence, rapid clinical deterioration.
Doxycycline is the treatment of choice for children and adults of all ages with suspicion of RMSF and is effective in prevention of more severe sequelae of this disease when administered within the first five days of symptoms. Chloramphenicol is the only alternative pharmacologic treatment that has been used to treat RMSF. Nonetheless, the epidemiological studies suggest that patients with RMSF treated with chloramphenicol have higher risk of death than persons who received a tetracycline.6 Chloramphenicol is associated with adverse hematologic effects, and close monitoring of blood levels is required with use of this medication 9
The long-term reevaluation of this patient revealed diffuse neurologic damage: cortex damage involving language and psychomotor components, which was significantly diminished in comparison with her state before infection. Motor sequelae were noted regarding spasticity in all four extremities as well as gait reflected spastic pattern.
The neurologic sequelae reported in the United States during the middle of the twentieth century included paraparesis, hearing loss, peripheral neuropathy, urinary and fecal incontinence, cerebellar, vestibular and motor dysfunction, as well as language delay.10
The variation in clinical manifestations between those reported and this patient is a product of lack of attention to neurologic sequelae, nutritional state, and other related complications, such as the spastic paraparesis progressed to spastic quadriparesis.
Neurologic sequelae related to RMSF have been described, and the treatment for them include rehabilitation in three different areas: speech therapy (with aphasia), physical therapy (for paralysis, paraparesis and spasticity), and gait rehabilitation (depending on severity). In the case of this patient, unfortunately, is possible that the socioeconomic status caused the lack of neurologic follow up, rehabilitation and therapy, as well as pharmacologic treatment, which together lead to inadequate neurologic recuperation.
The training of healthcare professionals is necessary and urgent, as the suspicion of RMSF together with appropriate and timely treatment are key in avoiding deaths and unnecessary complications of the disease.
The promotion of health and disease prevention constitute the core of public health. One fundamental element of health promotion is its anticipatory nature, which seeks out not the disease directly, but rather the social determinants of health. Without doubt, poverty and marginalization are factors that allow the vector to find its ideal host in order to continue reproducing and prevail. Training and empowering the population regarding self-care from diseases transmitted by vectors in endemic zones is the path to eradicating this disease.
We thank the researchers Fernando Puerto-Manzano and Travis Gordon for their valuable contributions in this case report.