Influenza is an important cause of morbimortality worldwide. Although people at the extremes of age have a greater risk of complications, influenza has been more frequently investigated in the elderly than in children, and inpatients than outpatients. Yearly vaccination with trivalent or quadrivalent vaccines is the main strategy to control influenza.
ObjectivesDetermine the clinical and molecular characteristics of influenza A and B infections in children and adolescents with influenza-like illness (ILI).
MethodsA cohort of outpatient children and adolescents with ILI was followed for 20 months. Influenza was diagnosed with commercial multiplex PCR platforms.
Results179 patients had 277 episodes of ILI, being 79 episodes of influenza A and 20 episodes of influenza B. Influenza A and B cases were mild and had similar presentation. Phylogenetic tree of influenza B viruses showed that 91.6% belonged to the B/Yamagata lineage, which is not included in trivalent vaccines.
ConclusionsInfluenza A and B are often detected in children and adolescents with ILI episodes, with similar and mild presentation in outpatients. The mismatch between the circulating influenza viruses and the trivalent vaccine offered in Brazil may have contributed to the high frequency of influenza A and B in this population.
Influenza (INF) A and B viruses are a frequent cause of respiratory tract infection in humans, affecting people of all ages with varied morbidity, sometimes leading to fatal outcomes. INF A viruses are divided into various subtypes based on the antigenic properties of surface glycoproteins, hemagglutinin and neuraminidase. INF B viruses are not subdivided into subtypes, but two genetically distinct lineages of influenza B viruses, namely B/Yamagata lineage and B/Victoria lineage have circulated globally since 1985 and co-circulated since 2001.1–3
People at the extremes of age are at increased risk of complications, hospitalization and influenza-associated death. The burden of influenza has long been recognized in the elderly due to the increased risk of death secondary to pneumonia or cardiac complications.4 In young and very young children, studies have demonstrated that the burden of influenza-associated acute lower respiratory infection (ALRI) is also substantial.5 A recent meta-analysis including 43 studies and data from approximately eight million children, estimated that 90 million new cases of influenza and 20 million cases of influenza-associated ALRI occurred worldwide in children younger than five years in 2008.6 However, few studies have considered the burden of influenza A and B in the outpatient setting.7
Seasonal influenza vaccination is the main strategy to prevent influenza and its complications. In Brazil, the trivalent influenza vaccine (TIV) is offered at no cost for individuals aged 60 years and over, children from six months to five years of age, pregnant and puerperal women (up to 45 days postpartum), health care workers, school teachers, indigenous populations, immunocompromised patients or with chronic conditions, adolescents and young adults aged 12–21 under socio-educational measures, and prisoners and prison staff. The quadrivalent vaccine (QIV), which includes also the B/Yamagata lineage is available only in private clinics. Influenza vaccination campaigns have been successful, with vaccine coverage above 70%. However, the impact of campaigns on reducing influenza burden is not well established since the information on the incidence and severity of influenza is limited. Data on the burden of influenza B in children and adolescents are even scarcer.
During a prospective cohort study of dengue in children and adolescents living in the city of Araraquara, Sao Paulo, Brazil the investigators observed that patients with fever and respiratory symptoms such as cough, coryza and sore throat were more likely to test negative for dengue.8 We then recognized in this consolidated cohort, a window of opportunity to prospectively compare the frequency, epidemiology and morbidity of influenza A and B infections in outpatient children and adolescents with influenza-like illness (ILI).
Material and methodsStudy population and sample sizeThe consolidated dengue cohort was recruited between August 2014 and March 2015. A total of 3,514 children and adolescents aged two to 16 years were randomly selected from the population of Araraquara at the time of recruitment. From December 2016 to August 2018, subjects from the original cohort who presented ILI were included in the influenza cohort and prospectively followed during two consecutive influenza seasons. In 2017 and 2018, the winter started on June 21st in the southern hemisphere. Sample size was estimated based on the frequency of febrile episodes with respiratory symptoms observed in the first year of the original (dengue) cohort. During the 20-month period of the study, we hypothesized that 1,200 cases of febrile syndrome followed by respiratory symptoms would occur, of which 5% would be influenza with virological confirmation.9 Thus, at least 60 cases of proven influenza infection would be available for analysis.
DefinitionsILI was defined by the presence of fever and two or more of the following signs and symptoms: cough, coryza, tonsillitis, pharyngitis, tachypnea, dyspnea, myalgia, headache, inappetence or prostration appearing within seven days. Proven influenza infection was defined by the occurrence of ILI with a laboratory confirmation of influenza A or B. Lymphopenia was defined as an absolute lymphocyte count (ALC) below 1.0×106 lymphocytes/mm3. Leukocytosis was defined as an elevated white blood cell (WBC) count greater than 11,000per mm3.
Follow-upAfter enrollment, parents or guardians received a weekly phone call for fever surveillance. In the case of a temperature >37.5°C and respiratory symptoms, a home visit was scheduled to confirm ILI and collection of oral and nasopharynx swabs. In addition to demographic data, the following information was assessed during home visits: type of respiratory symptoms, date of initial symptoms, presence of symptomatic household contacts, presence of comorbidities, need of hospitalization and information on influenza vaccination in that year. All information was transcribed to case report forms (CRF). Children with laboratory-confirmed influenza or with more pronounced symptoms were referred to a pediatrician by the field team. Oseltamivir was introduced at physician discretion.
Diagnosis of influenza and other respiratory virusesRespiratory virus diagnosis was performed at the Virology Laboratory, Institute of Tropical Medicine (University of São Paulo School of Medicine). Influenza A or B was diagnosed by nucleic acid test (NAT), using the Cepheid (Xpert® Xpress Flu, Sunnyvale, CA, USA) platform, or the XGen Influenza multiplex kit (Mobius Life Science, Pinhais, PR, Brazil) according to manufacturer’s instructions. Due to its rapid turnaround time, the Cepheid platform was used first, to assure early introduction of oseltamivir, at physician discretion. Aliquots of respiratory samples were frozen and stored at −80° C for further diagnosis of other pathogens using a multiplex platform (XGen Respiratory Panel 21 pathogens multiplex kit (Mobius Life Science, Pinhais, PR, Brazil), according to manufacturer’s instructions.
Sequencing of influenza B virusesSequencing was proposed to evaluate the match between circulating influenza B viruses and the lineages included in the trivalent (TIV) and quadrivalent (QIV) influenza vaccines recommended during the study. Primers for the hemagglutinin (HA) region were designed for INF B (FluBHA_F1-TTGGAACCTCAGGRTCTTGC or FluBHA_F2 – TCCTATAATGCACGAYAGAACA and FluBHA_R-TGCAGGAGGTCTATATTTGGTTC). Fragments were sequenced using Sanger method and sequences were built and analyzed in the CLC GenomicsWorkbench (Qiagen-https://www.qiagenbioinformatics.com/products/clc-genomics-workbench/). The visualization and obtaining of the information described in the report were done with the aid of the CLC. Reference sequences of Influenza vaccine strains were obtained from the GISAID - Global Initiative on Sharing All Influenza Data (https://www.gisaid.org/). These were aligned together to worldwide references samples from 2015 to 2018 available at GenBank and also to sequences generated in this study.
We first looked for amino acid differences between our samples and the vaccine strains. Then we reconstructed a phylogenetic tree from partial HA gene (47 sequences with 616 nucleotides long) to investigate the epidemiology of the circulating strains. Phylogenetic reconstructions were performed using the Maximum Likelihood method in the PhyML program within the SeaView package10 using the best nucleotide model (HKY+I) chosen by ModelTEst.11
Influenza vaccines and policiesIn 2016, the components of influenza vaccine for the southern hemisphere were A/California/7/2009 (H1N1)pdm09, A/Hong Kong/4801/2014(H3N2) and B/Brisbane/60/2008 (B/Victoria lineage). The strain A/Michigan/45/2015(H1N1)pdm09 replaced the strain A/California/7/2009(H1N1)pdm09 in 2017. The quadrivalent influenza vaccine (QIV) also including the lineage B/Phuket/3073/2013-like virus (B/Yamagata lineage) and was only available in private clinics.
Ethical issuesThe study was approved by the Ethics Committee of the Faculty of Medicine of the University of São Paulo (CAAE no. 25706913.6.1001.0065). The selected households were visited by the project’s field team. The parents or guardians were asked to sign additional informed consent forms, specific for the influenza study. The child and/or the adolescent were requested to sign the Term of Assent, in accordance with the current legislation.
Statistical analysisPatient characteristics were summarized using descriptive statistics. Differences between categorical and continuous variables were calculated using Pearson’s chi square, Student’s t-test and Mann Whitney tests, as appropriate. In all statistical analyses, a two-sided p-value≤0.05 was considered statistically significant (IBM SPSS statistics version 21 for Windows).
ResultsStudy populationThe study started on December 5, 2016, when the first case of ILI was included, and ended on August 30, 2018. Thus, the study cohort was followed for 20 consecutive months, including two winters. One hundred and seventy-nine patients were included, 99 males (55.3%) and 80 females (44.7%), median age 10 years, ranging from eight months to 19 years of age.
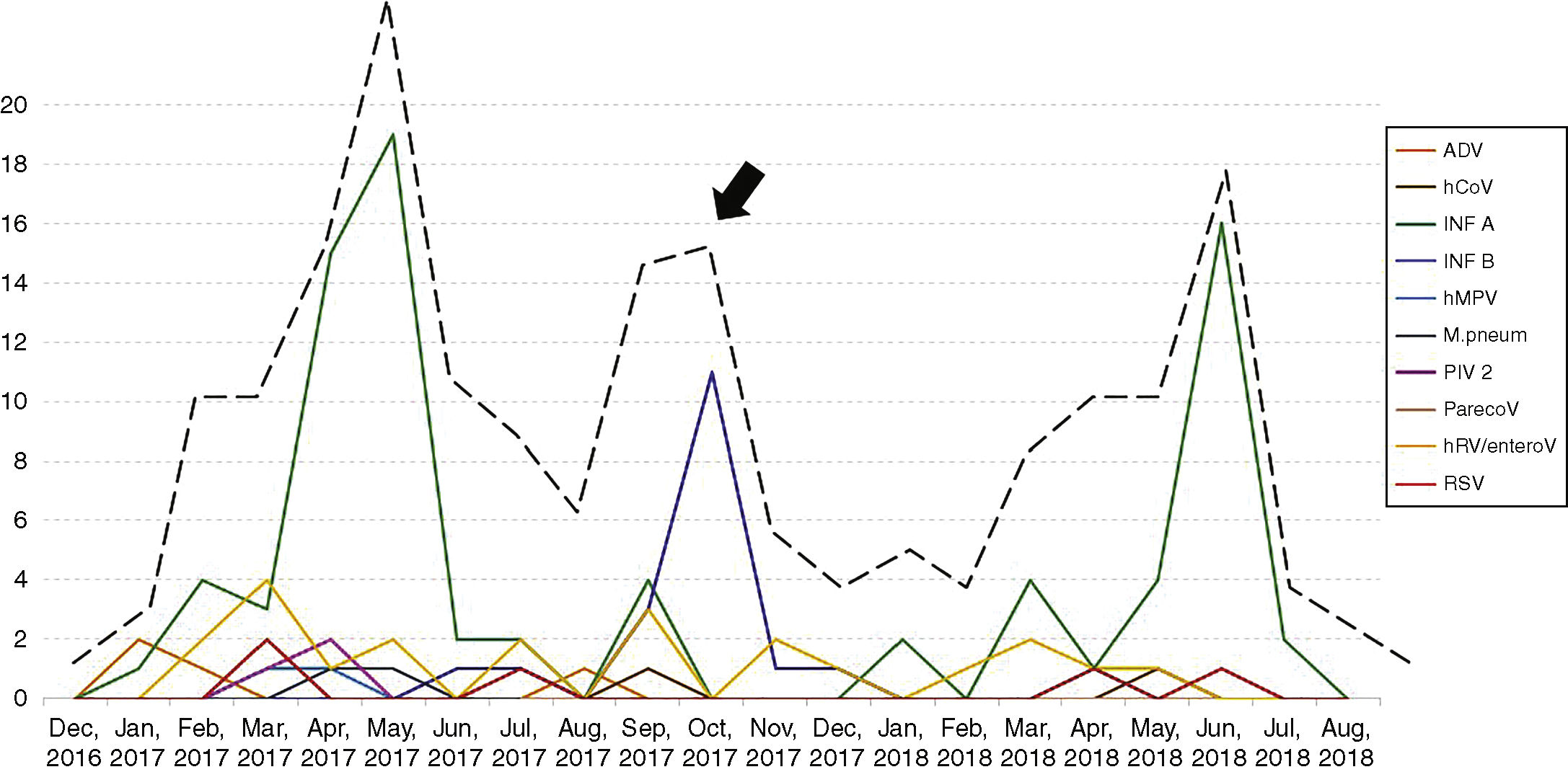
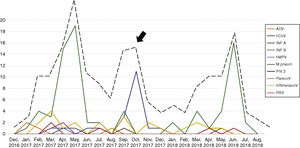
Seasonality and clinical findings in ILI episodesThe patients had 277 episodes of ILI during the study period, with a median of one episode per patient, ranging from one to four episodes. Thirty-three patients had two episodes of ILI, eight had three episodes and two had four episodes of ILI. As expected, an increase in the number of episodes of ILI was observed in winter months, although a second wave of ILI was observed in the spring of 2017 (arrow, Fig. 1). In addition to fever, the most frequent symptoms were headache, observed in 233 of the 277 ILI episodes (84.1%), somnolence (in 208 episodes, 75.1%), cough (in 204 episodes, 73.6%) and coryza (in 193 episodes, 69.7%). Hemogram was performed in 202 of the 277 episodes of ILI (73%). Median leukocyte and lymphocyte counts were 6,200 (1,800–31,300)/mm3 and 2,419 (708–4,602)/mm3, respectively. Leukocytosis was observed in 121 (60%) and lymphopenia in four (2%) of the ILI episodes. Children were referred to the pediatrician in 87 of the 277 episodes of ILI (31.4%). No patient with ILI needed hospital admission.
Laboratory-confirmed influenza A and BUsing the influenza platform (Cepheid Xpert® Xpress Flu) we identified 90 episodes of proven influenza infection, 73 influenza A (81.1%) and 17 by influenza B (18.9%). During laboratory work up with the multiplex platform to identify other respiratory pathogens, nine additional influenza infections were diagnosed (three influenza B and six influenza A). Thus, 90 of the 179 patients (50.3%) had 99 episodes of proven influenza infection, being 79 caused by influenza A (79.8%) and 20 by influenza B (20.2%). Nine patients had two episodes of influenza. Two patients had two episodes of INF A in the same year; four had two episodes of INF A in consecutive years; one patient had two episodes of INF B in the same year; and three patients had two separate episodes of influenza A and B in the same year (one patient), or in consecutive years (two patients).
In general, the clinical presentations of influenza A and B infections were similar and regularly mild, as shown in Table 1. Patients with influenza B were more likely to be male (85%, p=0.01) and presented more prostration than patients infected with influenza A virus (p=0.01). Among the 87 outpatient visits to the pediatrician, 61 were due to influenza (70.1%). Interestingly, the number of visits was significantly higher for INF A episodes in comparison with INF B (67.1% vs 40%; p=0.0003). Oseltamivir for five days was introduced only in one case of influenza A.
Clinical and laboratory findings in influenza A and B infection (N=99).
| Variable | Category | Influenza A | Influenza B | p Value |
|---|---|---|---|---|
| N=79 (%) | N=20 (%) | |||
| Median age (range) years | 9 (4–17) | 9 (5–14) | NS | |
| Sex | Female | 37 (37.4) | 3 (3.0) | 0.01 |
| Male | 42 (42.4) | 17 (17.2) | ||
| Symptoms | Coryza | 67 (84.8) | 20 (100) | 0.06 |
| Somnolence | 66 (83.5) | 19 (95) | 0.19 | |
| Inappetence | 62 (78.5) | 15 (75) | 0.74 | |
| Headache | 72 (72.7) | 18 (18.2) | 0.87 | |
| Cough | 71 (71.7) | 16 (16.2) | 0.23 | |
| Myalgia | 49 (62) | 13 (65) | 0.80 | |
| Abdominal pain | 46 (58.2) | 9 (45) | 0.23 | |
| Nausea | 46 (58.2) | 8 (40) | 0.14 | |
| Prostration | 38 (48.1) | 16 (80) | 0.01 | |
| Conjunctivitis | 30 (38) | 12 (60) | 0.075 | |
| Arthralgia | 30 (38) | 5 (25) | 0.28 | |
| Pharyngitis | 25 (31.6) | 5 (25) | 0.56 | |
| Vomit | 24 (30.4) | 6 (30) | 0.97 | |
| Tonsillitis | 19 (24.1) | 4 (20) | 0.70 | |
| Diarrhea | 16 (20.3) | 4 (20) | 0.98 | |
| Dyspnea | 12 (15.2) | 3 (15) | 0.98 | |
| Rash | 5 (6.3) | 2 (10) | 0.56 | |
| Lethargy | 3 (3.8) | 2 (10) | 0.26 | |
| Tachypnea | 1 (1.3) | 1 (5) | 0.29 | |
| Median lymphocyte count (range) | 2,343 (708–4,602) | 2,743.5 (1,888–4,484) | 0.89 | |
| Leukocytosis | 30 (38) | 4 (20) | 0.18 | |
| Lymphopenia | 4 (5) | 0 (0) | 0.58 | |
| Outpatient visits | 53 (67.1) | 8 (40) | 0.026 | |
One hundred and thirty-eight of the 277 episodes of ILI (49.8%) tested negative by all techniques. In the remaining 139 episodes (50.2%), at least one respiratory pathogen was identified. Influenza A and B viruses were the most frequent agents identified (99 episodes) followed by human rhinovirus (22 episodes). Table 2 shows the respiratory pathogens diagnosed during the study. Fig. 1 shows the seasonality of ILI and of the respiratory pathogens. The second wave of ILI episodes observed in 2017 was mainly due to influenza B infections.
Respiratory pathogens identified in 139 episodes of influenza-like illness.
| Pathogen | Number (%) |
|---|---|
| Influenza A | 76 (54.6) |
| Influenza B | 20 (14.4) |
| Rhinovirus | 16 (11.5) |
| Influenza A/H1N1 | 2 (1.4) |
| Influenza A+Rhinovirus | 1 (0,07) |
| Rhinovirus+enterovirus | 4 (2.8) |
| Rhinovirus+RSV | 1 (0.07) |
| Respiratory Syncytial Virus | 4 (2.8) |
| Adenovirus | 4 (2.8) |
| Parainfluenza 2 | 2 (1.4) |
| Parainfluenza 2+metapneumovirus A/B | 1 (0,07) |
| Metapneumovirus A/B | 2 (1.4) |
| Human coronavirus | 2 (1.4) |
| Mycoplasma pneumoniae | 2 (1.4) |
| Parecovirus | 2 (1.4) |
Information on influenza vaccination was obtained during home visits, when respiratory samples were taken. In 49 of the 277 episodes of ILI (17.7%), the information about influenza vaccine was not available. In the remaining 228 episodes of ILI, 85 had received the trivalent vaccine (37.3%), and 143 had not been vaccinated (62.7%). Excluding the cases without vaccine information, no difference was seen in the occurrence of influenza in vaccinated and non-vaccinated patients (p=0.47). According to the country recommendation, seven children were within the age range of influenza vaccine. In two of them, the vaccination status was unknown, and out of the remaining five, four (80%) had been vaccinated.
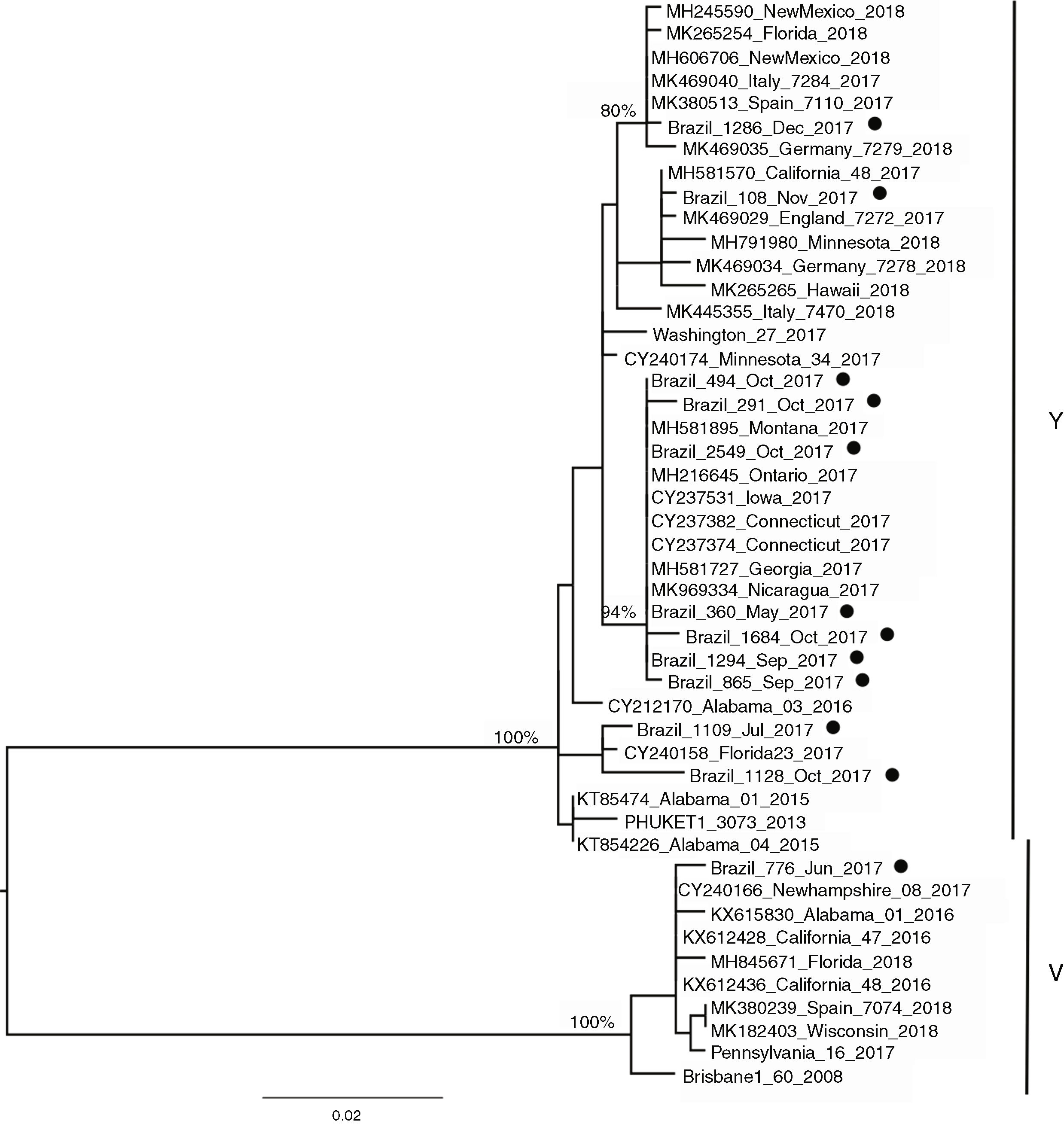
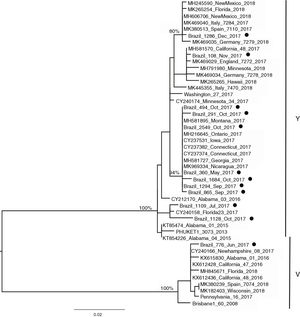
Influenza B lineagesFrom the 20 cases of influenza B, 12 sequences could be generated and were used for analysis. The amino acid analysis showed that while 11 strains were related to the Phuket (B/Yamagata lineage), one of the circulating virus (patient BR_776_June_2017) was similar to the Brisbane virus (Victoria lineage) (data not shown). This was also noticeable through the phylogenetic reconstruction (Fig. 2) where the two main clusters comprised strains related to either Brisbane or Phuket lineages sampled at the same location and years, indicating co-circulation of both strains. The phylogeny also revealed that there was little difference among the Brazilian, European and USA circulating influenza B viruses since they all clustered together with no particular geographic pattern. A slight temporal structure can be observed in the tree, with viruses sampled in September and October clustering together, and samples from November and December 2017 clustered together to viruses sampled in 2018 (from other countries). In comparison to the vaccine lineages (Brisbane_60 and Phuket_3073), we observed that six of the eleven patients (54.5%) in the Phuket branch of the phylogenetic tree had not received influenza vaccine, one (9%) had unknown vaccination status, and four (36.3%) had received the TIV, which contained only the Brisbane lineage of influenza B virus. The only patient with influenza B in the Brisbane branch had not been vaccinated.
DiscussionAs the dengue epidemic subsided in the city of Araraquara, we detected fewer febrile episodes in the following years than anticipated for the influenza cohort. However, this fact did not affect the expected number of influenza cases to be analyzed in the study. We observed a high frequency of influenza A and B in children and adolescents with fever and respiratory symptoms. Among the 277 episodes of ILI, 99 (35.7%) were caused by influenza viruses, mostly influenza A (79.9%). The use of “influenza-like illness” as the case definition for respiratory sampling justifies this finding. Similar rates were found in a recently published study that detected 263 virologically confirmed episodes of influenza in 811 ILI episodes (36.7%), the majority also caused by influenza A viruses.12 In three consecutive influenza seasons, other authors detected 48% of influenza in ILI episodes in children and adolescents aged five to 14 years, a similar age range of the present cohort.13 Additionally, the use of robust diagnostic tests in multiplex platforms enhanced the likelihood of influenza diagnosis due to superior sensitivity of these new assays.14
It is well established that INF A and INF B co-circulate annually. In the USA, the proportion of circulating INF A and INF B viruses has varied from 65% to 88% and from 12% to 35%, respectively, in the last 10 years.15 Our findings (79.9% of INF A and 20.1% of INF B) reflect co-circulation of influenza A and B viruses in Brazil during the study period. In 2016, the proportion of INF A and B viruses was 69.7% and 30.3%, respectively; 62% and 38% in 2017; and 80.1% and 19.9% in 2018.16–18 It is difficult to predict which influenza virus will predominate in a given season. In the southern hemisphere spring of 2017, a second wave of ILI related to influenza B infections was observed. Influenza surveillance data from the Brazilian Ministry of Health showed that in 2016 and 2017, influenza B viruses circulated with greater activity in spring months. As early as 2018, this trend did not remain.16–18 In other countries, this second wave of INF B viruses were also observed in the season.13
Concerning to the clinical findings, symptoms caused by influenza B virus were remarkably similar to those caused by influenza A virus, and included fever, headache, cough, somnolence, myalgia, abdominal pain, vomiting and conjunctivitis, as observed by other authors.19,20 We found a higher proportion of males among INF B infections, which also caused more prostration than INF A infections. Few studies have shown that male sex may predispose to respiratory virus infection. A recent observational study has shown a significant association between male sex and adenovirus infection, but not with influenza or other respiratory viruses.21
Early beliefs were that influenza B viruses caused less severe disease than influenza A viruses.22 Taking into account the hospitalization rates, morbidity or mortality of influenza viruses over the last decades, INF B infections are now considered less severe than those caused by A/H3N2 viruses, but more severe than A/H1N1 infections.15,19,23 Both influenza A and B viruses may cause rare neurological complications such as encephalitis and Reye’s syndrome, the latter more often associated with INF B infections.19 From 2010 to 2018, the frequency of INF B infections associated with pediatric deaths varied from 15% to 37% in the USA.15 In Brazil in 2017, 498 deaths of patients with severe acute respiratory syndrome (SARS) were due to influenza viruses, the vast majority caused by influenza A(H3N2) (277 deaths, 55.6%) and influenza B (154 deaths, 30.9%).17
Fortunately, in the present cohort no patient died or had severe influenza requiring hospitalization. Maybe individuals included in prospective cohort studies are more properly informed about the importance of early report of symptoms and advised to seek medical attention sooner than the general population.
Although we did not categorize the burden of ILI episodes, the frequency of visits to pediatricians was 70.1% in the case of influenza infections in comparison with 29.9% in other ILI episodes (p<0.0001). As much as 57% of outpatient visits have been reported in laboratory-confirmed influenza episodes.12 In the present study, patients with INF A were more frequently referred to the pediatrician (67.1%) than those with INF B (40%). The above-mentioned belief that INF A is more severe than INF B may explain this finding.
We also did not observe any difference in the frequency of influenza infections among vaccinated and non-vaccinated patients (34% vs 40%, p=0.47). Seasonal influenza vaccination is the most effective way to prevent influenza and its complications.24 However, due to the changing nature of the influenza virus, influenza vaccine effectiveness (IVE) may vary over different influenza seasons and virus subtypes/lineages. A recent study conducted in the Netherlands from 2004 to 2014 showed that the adjusted IVE dropped from 40% when the vaccine (partially) matched the circulating viruses, to 20% in mismatched seasons. The authors also observed that IVE was particularly low when A(H3N2) was the predominant influenza virus subtype.25
Concerning to influenza B lineages, a review of INF B infections conducted in 26 countries showed that a vaccine mismatch occurred in approximately 25% of the seasons.26 In a mismatched season, IVE may be suboptimal against INF B, potentially increasing the burden of the disease.2 In a recently published study, conducted in the state of Paraná, the authors observed that both B/Yam and B/Vic lineages co-circulated from to 2013 to 2016, with a frequency of 47% and 53%, respectively.27 In Brazil, previous studies have demonstrated the mismatch between vaccine and circulating lineages of influenza B.28
We observed a higher proportion of the B/Yamagata lineage circulating in the city of Araraquara during the study period. As this lineage was not included in the TIV provided by the Brazilian Ministry of Health, protection against INF B infections was certainly lower than expected. Among the 10 INF B/Yamagata lineage patients with known vaccination status, four (40%) had received the TIV and six (60%) had not been vaccinated.
In the 12 cases of INF B that could be sequenced, one case of INF B/Victoria lineage was detected in a 5-year-old child. In the remaining 11 (median age 10, range six to 12 years) we identified the B/Yamagata lineage. Recent epidemiological studies have demonstrated that viruses of the B/Victoria lineage infect subjects at a relatively younger age than those of the B/Yamagata lineage.29,30 As both INF B lineages have been circulating since 2001, and it is widely recognized that the QIV vaccine offers better protection than the TIV,19 it sounds paradoxical that to date there is no recommendation of preferential use of QIV over TIV.
In conclusion, our study showed that influenza viruses are often detected in children and adolescents during ILI episodes. Morbidity of INF A and B infections was very similar, and therefore it is time to overcome old beliefs that INF A is more severe than INF B. The mismatch between the circulating INF viruses and the TIV offered in public health services in Brazil may also have contributed to the high frequency of influenza A and B in this population.
This study was supported by Sanofi-Aventis Farmacêutica Ltda (Sanofi FLU 55), contract number 104225 (FFM-USP). The authors thank Bárbara B. de Souza Pereira, Marta Inenami, Raphael Luiz de Holanda e Silva, Eliezer Robson, Mariana Cristina Câmara, Fernanda de Jesus Notário dos Santos and Maísa de Fátima Hortenci Corona for laboratory support and field assistance during the study.