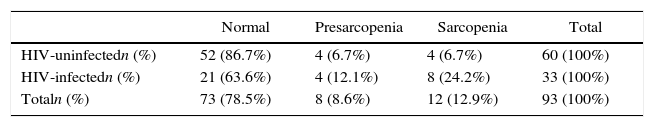
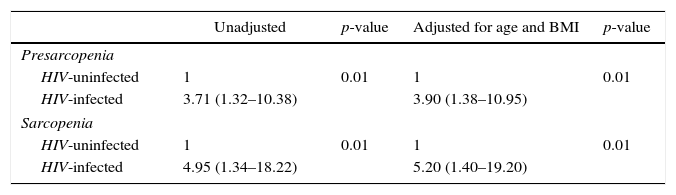
Presarcopenia and sarcopenia were evaluated in HIV-infected individuals and in healthy elderly controls according to the consensus definitions of the European Working Group on Sarcopenia in Older People. Bioelectrical impedance, a hydraulic hand dynamometer, and gait speed were used to evaluate muscle mass, muscle strength, and physical performance, respectively. Adjusted and unadjusted binary logistic regression predicted the risk of sarcopenia. Predictor contribution was assessed by the Wald test. Significance was established at p≤0.05. The HIV-infected group consisted of 33 patients on treatment (42.4% women; mean age 59±7 years; mean BMI 25±6kg/m2; viral load undetectable in 30 cases). The HIV-uninfected group consisted of 60 individuals (71.7% women; mean age 70±7 years; mean BMI 28±6kg/m2). Of the controls, 4 (6.7%) individuals had presarcopenia and 4 (6.7%) sarcopenia compared to 4 (12.1%) and 8 (24.2%), respectively, in the HIV-infected group. The HIV-infected patients had a 4.95 higher risk (95% CI: 1.34–18.23) for sarcopenia compared to the controls. It should be pointed out that the control group was on average 10 years older. This risk increased further (RR=5.20; 95% CI: 1.40–19.20) after adjusting for age and BMI. HIV-infected patients were shown to be at a greater risk of sarcopenia, an indicator of frailty, even following adjustment for age and BMI.
The term sarcopenia (sarx meaning flesh and penia poverty) was first proposed by Irwin Rosenberg in 1989 to describe the decline in muscle mass associated with age.1,2 Currently, the European Working Group on Sarcopenia in Older People (EWGSOP) has broadened this concept by including muscle strength and physical performance as additional diagnostic criteria, and proposing the stages presarcopenia, sarcopenia, and severe sarcopenia.3
Human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) is currently considered a chronic disease due to the advances made in antiretroviral therapy (ART) in recent years. As the prevalence of opportunistic infections decreased, there was an increase in the prevalence of chronic pathologies such as cardiovascular, liver, and kidney diseases, cognitive disorders, and osteoporosis.4 This fact has generated speculations in relation to a probable “accelerated aging syndrome” in HIV-infected patients.5 Several investigators have demonstrated bone mass loss and a greater fracture risk in HIV-infected patients.6 Whenever premature age-related comorbidities are detected in HIV-infected patients, it is crucial to evaluate the presence of sarcopenia, an important condition responsible for an increased risk of falls and fractures that may ultimately lead to immobility and dependency.7 Indeed, sarcopenia may increase morbidity and mortality.8
The objective of this cross-sectional, analytical study was to compare the prevalence of sarcopenia, presarcopenia, and severe sarcopenia in HIV-infected patients compared to healthy HIV-uninfected elderly individuals.
The study was conducted at the sexually transmitted infections/AIDS outpatient clinic and at the geriatric outpatient clinic of the Santa Casa de Misericórdia, a university teaching hospital in Vitória city, Brazil between December 2013 and July 2014. The sample consisted of HIV-infected individuals of 50 years of age or more under antiretroviral therapy (ART) and HIV-uninfected individuals of 60 years of age or more. Individuals with neurological diseases, chronic pulmonary disease, partial paralysis, or any other morbidity that could affect their physical performance were excluded from the study. The study protocol was approved by the internal review board of the Escola Superior de Ciências da Saúde da Santa Casa de Misericórdia de Vitória (EMESCAM) under reference CAAE: 09180512.9.0000.5065. All participants signed an informed consent form.
A bioelectrical impedance scale (InBody 520 device; Biospace Inc., Beverly Hills, CA, USA) was used to evaluate muscle mass. The reproducibility of this method is good and it represents an acceptable alternative to the gold standard tests, magnetic resonance imaging and dual-energy X-ray absorptiometry (DXA).9 Using the Janssen equation, the normalized skeletal muscle index (SMI) (absolute muscle mass in kilograms/squared height in meters) was calculated. SMI was defined as low when values were ≤10.75 for men and ≤6.75 for women.3,10
Muscle strength was evaluated using a Jamar hydraulic hand dynamometer (Sammons Preston, Bolingbrook, IL, USA). The arithmetic mean of three measurements taken with the dominant hand was calculated and then adjusted for body mass index (BMI).3 Muscle strength was considered reduced under the following conditions for men: BMI≤24 and strength≤29kg; BMI from 24.1 to 28 and strength≤30kg; BMI>28 and strength≤32kg. The parameters for women were: BMI≤23 and strength≤17kg; BMI from 23.1 to 26 and strength≤17.3; BMI from 26.1 to 29 and strength≤18kg, and BMI>29 and strength≤21kg.11 These parameters were previously defined and validated by the European working group on sarcopenia in older people.12
Physical performance was evaluated according to the gait speed test in which the patient is asked to walk a distance of 4m in a maximum time of 5s, with performance being considered impaired when speed is less than 0.8m/s3.
In accordance with the EWGSOP definitions, low SMI as an isolated finding characterized presarcopenia. The association of low SMI with either low muscle strength or poor physical performance was considered sarcopenia, while a reduction in all three criteria was classified as severe sarcopenia.3
The continuous variables were described as means and standard deviations, while the dichotomous variables were described as percentages. Student's t-test for independent samples and chi-square test or Fisher's exact test were used for group comparison. Binary logistic regression, either unadjusted or adjusted for age and BMI, was used to predict the likelihood of association with sarcopenia. The statistical significance of the contribution of the predictors in the regression model was determined using the Wald test. p-values<0.05 were considered statistically significant. The SPSS statistical software program, version 22.0 was used throughout the analysis.
The study was conducted over a 6-month period and included 93 individuals. Of these, 33 were HIV-infected patients on ART. In 30 (90.9%) of those patients, viral load was undetectable. In relation to gender, 42.42% were women and 57.58% men. Mean age was 59±7 years (range 50–78 years) and mean BMI was 25±6kg/m2 (range 17.7–52.4kg/m2). Of the 60 HIV-uninfected controls, 71.7% were women and 28.3% men. Mean age was 70±7 years (range 60–87 years) and mean BMI was 28±6kg/m2 (range 15.3–41.6kg/m2).
Based on the EWGSOP diagnostic criteria for sarcopenia, 52 individuals in the HIV-uninfected control group (86.7%) were normal, 4 (6.7%) had presarcopenia and 4 (6.7%) had sarcopenia. In the HIV-infected group, 21 (63.6%) were normal, while 4 (12.1%) had presarcopenia, and 8 (24.2%) had sarcopenia (Table 1). Presarcopenia was 3.71 (95% CI: 1.32–10.38) times more common in the HIV-infected group in relation to the control group. After adjusting for age and BMI, this risk ratio increased to 3.90 (95% CI: 1.38–10.95). The risk of sarcopenia was 4.95 (95% CI: 1.34–18.23) times higher for HIV-infected individuals compared to the HIV-uninfected controls. Following adjustment for age and BMI, this risk ratio increased to 5.20 (95% CI: 1.40–19.20), as shown in Table 2.
The study group was younger and mostly male reflecting the composition of the HIV outpatient clinic whereas the control group was older and mostly female reflecting the composition of the outpatient geriatric clinic, as those individuals were consecutively recruited for the study protocol. As sarcopenia is more common in women than in men, and in older people than younger, we should have expected greater frequency of sarcopenia in the control group but the results have shown the opposite, even after adjustment by multivariate analysis. Unfortunately, as the sample was small, it was not possible to stratify the different groups for gender. The patients in the study group were on antiretroviral therapy for 7.15±3.74 years. The main backbone was Lamivudine–Zidovudine used by 17 patients followed by Lamivudine–Tenofovir in 16 patients. The third drug used was Efavirenz in 13 patients, followed by ritonavir-boosted Atazanavir in 8, ritonavir-boosted lopinavir in 8, nevirapine in 2, and ritonavir-boosted darunavir in the remaining 2 patients.
These findings reveal a strong positive association of presarcopenia and sarcopenia with HIV infection in patients with a mean age of 59 years and on regular use of ART, most with an undetectable viral load, compared to an uninfected group of older individuals with a mean age of 70±7 years of age. Although the mean age and gender distribution of patients that compose the two outpatient groups were not equivalent, that makes indeed stronger the association between HIV infection and sarcopenia. Yarasheski et al. conducted a longitudinal study and reported similar rates of skeletal muscle mass loss in an HIV-infected group and in the control group; however, those investigators failed to evaluate muscle strength and function.13
The present frequencies of presarcopenia and sarcopenia found for the HIV-infected patients (12.1% and 24.2%, respectively) were comparable to the rates of 20% and 5%, respectively, found in a cross-sectional study with a sample of HIV-infected individuals. In both studies, a high risk of sarcopenia in infected individuals was evident.11
Recently, Rees et al. reported a prevalence of frailty of 19% in 122 HIV-infected patients evaluated using the 5-component Fried frailty criteria. The most common criteria were depression and low physical activity. Curiously, the least common were the markers of sarcopenia, leading the authors to conclude that frailty in HIV-infected individuals is potentially reversible.14 Önen et al. evaluated a larger group of 445 HIV-infected patients with a mean age of 41.7 years and reported a prevalence of frailty of 9%, which was associated with comorbidities and more advanced immunodeficiency.15 The introduction of ART was found to exert a protective effect against frailty in the follow-up of the Multicenter AIDS Cohort Study in the United States.16
Wasserman et al. also reported a high prevalence of low muscle mass (between 18.8 and 21.9% depending on the definition used) in midlife and older HIV-infected individuals, particularly males, despite CD4 cell reconstitution and viral suppression.11 Several investigators have shown that HIV-infected patients experience a loss of bone mass and have a greater risk of fragility fractures.6,17 Until recently, the principal focus has been on preventing and managing bone disease in HIV/AIDS.18 Nevertheless, muscle mass loss has begun to attract the attention of healthcare providers, and there may be a correlation with bone mass loss. This may constitute one more risk factor for patients with HIV/AIDS.19
There are some clear limitations associated with the present study, particularly with reference to the small sample size and the consequent lack of statistical power to detect differences when comparing the frequency of sarcopenia between males and females or the loss of muscle mass with the use of different antiretroviral drugs. Nevertheless, the present results show a positive association of sarcopenia in HIV-infected individuals. This association remained positive even when compared with an older group and after controlling for the effect of age and BMI, thus constituting one more component in the risk of falls and fractures in the aging HIV-infected population.
Conflicts of interestThe authors declare no conflicts of interest.
Dr Alvaro Armando Carvalho de Moraes who helped with bioimpedance measures, and CNPQ and FAPES for funding this research.