HIV-infected children surviving until adulthood have been transitioning to adult outpatient health care service in Brazil since the late 2000's. Deterioration of clinical condition is expected during this period, as reported among youths with non-communicable chronic diseases. Despite their young age, they are long-term hosts of the virus, have prolonged exposure to antiretroviral therapy and have suffered from the social determinants and stigma of HIV infection since early childhood.
ObjectivesThis study aimed to 1) describe demographic and clinical characteristics at the first appointment at adult care service following pediatric care of a cohort of Brazilian youths living with HIV since childhood; and 2) retrospectively address adherence and clinical variables in the last two years of pediatric follow-up.
MethodsDescriptive study.
Results41 consecutive patients referred to adult outpatient care from a pediatric HIV unit were enrolled, median age 19 years, and median lifetime CD4+nadir 117 cell/mm3; 89% reported previous AIDS-defining conditions. At first laboratory assessment in adult care, only 46% had undetectable (<400 copies/ml) HIV viral load and the median CD4+count was 250 cell/mm3.
ConclusionYouths living with HIV at the transition from pediatric to adult care had poor treatment adherence, low lifetime CD4+cell nadir, low CD4 cell count and detectable HIV viral load. Health care providers should closely monitor these adolescents in a youth friendly environment, prepared for open communication about all aspects of their health.
HIV-infected patients who acquired the infection perinatally or in early childhood and survived to adult age have been transitioning to adult care in recent years. In Brazil, the first cohorts were referred to adult care in the late 2000's.1 The complex period of transition to adult care is often associated with deterioration of clinical condition, which has been reported among youths with non-communicable chronic diseases, such as type I diabetes, cystic fibrosis, rheumatoid arthritis, congenital heart diseases, sickle cell anemia, and chronic renal disease.2 Insufficient knowledge about their own health conditions, difficulties in self-care management, and treatment fatigue contribute to the hindering of disease control and increased morbidity and mortality during this transitional period.2
Youths living with HIV share several of these characteristics but also present particular features in dimensions that range from host-pathogen biologic interactions to the social determinants of the epidemic.3 Despite their young age, they are long-term hosts of the virus and therefore have prolonged exposure to antiretroviral therapy (ART). Adherence to therapy is often a challenge in this population, leading to impaired immune reconstitution and high occurrence of HIV-resistance mutations.3–7 Comorbidities such as cardiovascular diseases,8 metabolic conditions,9–12 and psychological or neurocognitive dysfunctions13–15 are also frequent in this population. Moreover, patients face the social stigma of HIV infection and remarkable challenges to lead their sexual lives,1 in addition to family or social adversities, rendering this group particularly vulnerable to unfavorable outcomes in clinical care.3,16–18
The influx of a large number of patients with such complex issues into adult HIV outpatient care may yield a significant burden to these specialized health services. A deeper understanding of the clinical characteristics and special needs of this group at transition to adult care could improve strategic allocation of resources aiming at a more successful disease control.
In this study, we describe demographic and clinical characteristics of a cohort of 41 Brazilian youths at transition from pediatric to adult outpatient care.
All participants underwent a similar clinical management protocol, following guidelines of HIV treatment for children and adolescents issued by the Brazilian Ministry of Health.19–21 These protocols have been sequentially adapted reflecting new scientific discoveries, incorporation of knowledge, and the increasing availability of ART drugs.
MethodsStudy design and populationAll consecutive youths living with HIV since childhood who were referred from pediatric to adult HIV outpatient clinics at our institution between January 2001 and December 2012 were enrolled. Since 2009, all patients transferred from pediatric care were individually reported with a complete medical history jointly revised by the pediatric and adult HIV clinicians. Patients transferred between 2001 and 2009 were identified using administrative registries from adult and pediatric clinics to identify those with appointments at both facilities. Patients who, despite referral, did not have an actual appointment at the adult outpatient clinic were excluded from the analysis.
The adult outpatient clinic comprises adult HIV care as well as teaching services for medical students and fellows in Infectious Diseases, psychology, and social service, with approximately 3,000 patients under care at the time these data were collected. Participants were referred from the pediatric HIV clinic located in a different facility of the same institution.
Study variablesData were collected from pediatric and adult medical records, laboratories, and administrative systems. The researchers used standardized forms to collect the following information from the records: age at first visit to the adult care service (years); sex; race (categorized as white vs. others); orphanhood; type of caregiver; source of infection; schooling years; occupation (categorized as no occupation; student only; employed; student and employed); sexual health history (sexual activity status and previous or current pregnancies); results of laboratory tests (CD4+counts and HIV viral load - VL) performed at the last visit and two years previous to the last visit at the pediatric follow-up and at the first visit to the adult outpatient care service (HIV VL considered undetectable when<400 copies/ml - 1.6 log10/mL); lifetime CD4+T cell nadir (CD4+nadir); time interval from CD4+nadir to the first visit of the adult outpatient care service [months]; treatment characteristics (time under ART [in years], current ART regimen, number of previous ART regimens [categorized as ≤4 or>4 previous regimens], number of daily doses and number of pills [categorized as<4 or ≥4 pills per day] in the current ART regimen); previous HIV genotypic resistance tests; AIDS-defining conditions according to CDC classification 22,23 during pediatric follow-up; AIDS-associated morbidities, hospitalizations, and comorbidities during the 24 months prior to referral to adult care (recorded as reported in medical charts during routine care, as well as according to prescribed medical and behavioral interventions); treatment adherence (assessed using 1. medical charts; 2. missed appointments with healthcare provider, and 3. records of ART dispensation at pharmacy). Poor adherence was defined as having any of the following: description of inadequate/low adherence in medical records; ≥25% missed medical appointments;<85% dispensations of ART regimens from the institutional pharmacy at timely schedule (dispensations compatible with adequate ART uptake and restock).
Statistical analysisDescriptive analysis was performed using frequencies and percentages, medians and interquartile ranges (IQR) for categorical and numeric variables, respectively. Incidence rates of AIDS-associated diseases and hospitalizations were calculated, as incidence density per 100 person-years, for the 24 months prior to admission in the adult care. Box plots were used to explore CD4+counts and HIV VL according to adherence to ART. All analyses were performed using Stata 14.2 (StataCorp. College Station, TX: StataCorp LP).
Ethical issuesOur Institutional Ethics Review Board approved this study (protocol 336.267). All patient identifiers were kept confidential.
ResultsForty-one youths reached adult care service between 2001 and 2012, 90% of them from 2009 to 2012. Main demographic characteristics are described in Table 1. A total of 39 (95%) participants were infected through mother-to-child transmission (MTCT) and 2 (5%) through blood transfusion, both before the age of five during leukemia treatment; both had remission of the hematologic disease reported in medical charts before HIV was diagnosed.
Demographic characteristics of 41 HIV infected youths at transition to adult outpatient care at a teaching tertiary care hospital, Sao Paulo, Brazil, 2001 – 2012.
| Variable | n (%) | Median (IQR) |
|---|---|---|
| Age – years | 19 (18 – 20) | |
| Female | 22 (54) | |
| White | 30 (73) | |
| Education - schooling years | 12 (11 – 12) | |
| MTCT | 39 (95) | |
| Orphan | 21 (51) | |
| Caregiver | ||
| Family member | 35 (85) | |
| Birth parent | 17 (48) | |
| Foster parent | 2 (6) | |
| Grandparent/other relative | 16 (46) | |
| Institution | 5 (12) | |
| None | 1(2) | |
| Occupation | ||
| Student | 11 (27) | |
| Employee | 10 (24) | |
| Student and employee | 8 (20) | |
| None | 12 (29) | |
MTCT – Mother-to-child transmission. IQR – interquartile range.
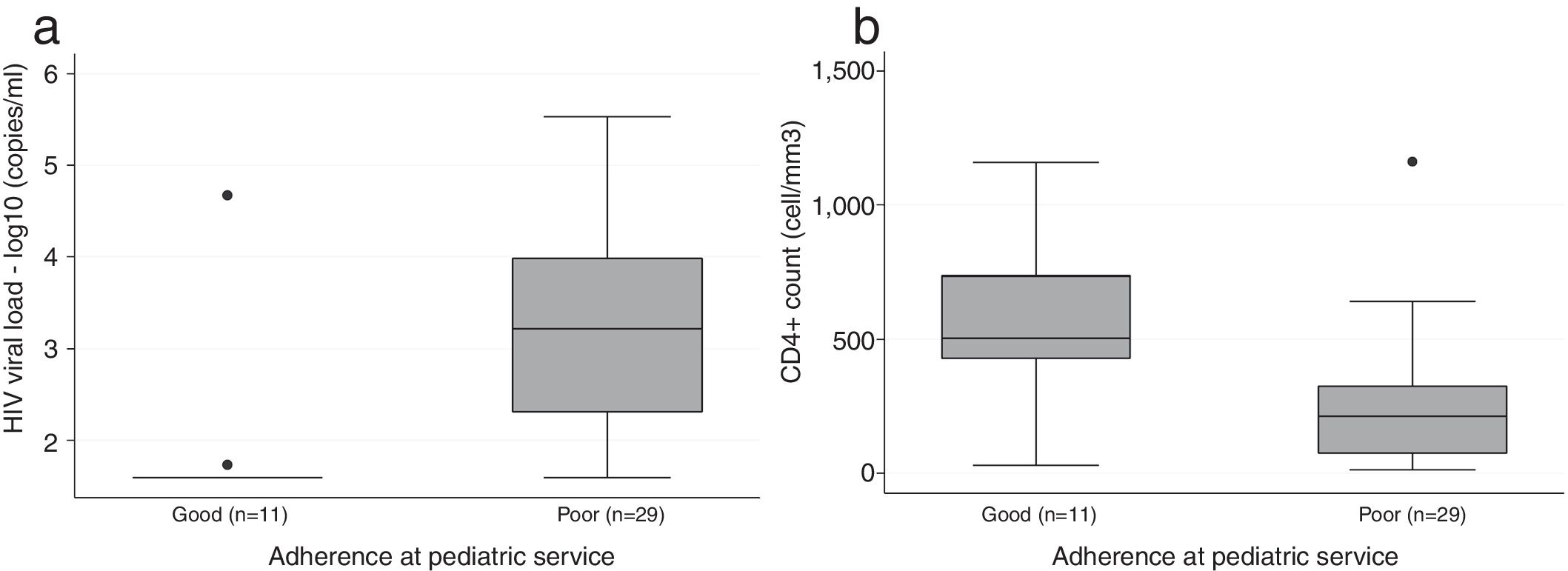
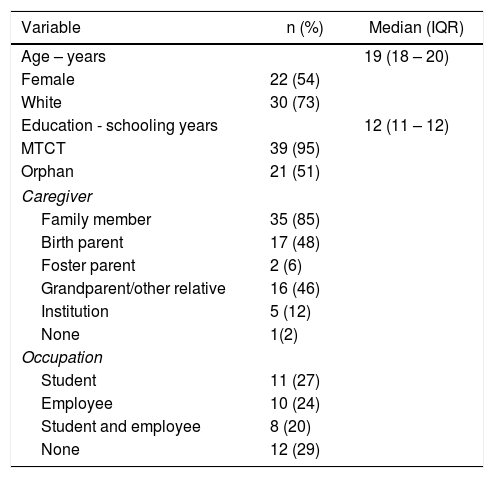
Clinical characteristics are summarized in Table 2. Of note, most participants had a previously impaired immune status, as shown by a low median lifetime CD4+cell nadir and a high proportion of patients with pediatric CDC classification 22B2 or worse; median time between CD4 cell nadir and the first visit to the adult outpatient clinic was 25 months. In laboratory tests collected at the first visit at adult outpatient care, only 46% had undetectable HIV VL, median CD4+count was 250 cell/mm3 (IQR 94-460), and 75% had CD4+counts below 500 cell/mm3. Among the 25 (54%) youths with a detectable HIV VL, median HIV VL was 3.83 log10 copies/mL (IQR 3.08-4.37). As shown in Figure 1, the group with poor adherence had lower median CD4+count and higher HIV VL compared to the group with good adherence. Metabolic conditions, dyslipidemia in particular, accounted for the most frequent comorbidities. Only one patient in the cohort had abnormal cognitive-motor development, reported as learning deficit and impaired self-care in pediatric medical charts.
Clinical characteristics of 41 HIV infected youths at transition to adult outpatient care in a teaching tertiary care hospital, Sao Paulo, Brazil, 2001 – 2012.
| Variable | n (%) | Median (IQR) |
|---|---|---|
| CD4 count (cell/mm3) | 250 (94 – 460) | |
| HIV viral load (copies/mL) | 2.74 (1.59 – 3.92) | |
| Undetectable (< 400 copies/mL) | 19 (46) | |
| Detectable (≥ 400 copies/mL) | 21 (51) | |
| Ignored | 1 (2) | |
| Paediatric CDC AIDS-classification | ||
| A1 | 1 (2) | |
| A2 | 3 (7) | |
| A3 | - | |
| B1 | 1 (2) | |
| B2 | 6 (15) | |
| B3 | 7 (17) | |
| C1 | - | |
| C2 | 2 (5) | |
| C3 | 21 (51) | |
| Lifetime CD4 nadir (cells/mm3) | 117 (30 – 237) | |
| Time between CD4 nadir and enrolment (months) | 25 (9 – 69) | |
| AIDS-related diseases at pediatric transition period | ||
| Tuberculosis | 5 (12) | |
| Herpes zoster | 3 (7) | |
| Oral candidiasis | 3 (7) | |
| Esophageal candidiasis | 2 (5) | |
| CMV meningoencephalitis | 1 (2) | |
| Hodgkin lymphoma | 1 (2) | |
| Herpetic paraparesis | 1 (2) | |
| Recurrent pneumonia | 1 (2) | |
| Hospitalization | 9 (22) | |
| Comorbidities | ||
| Dyslipidemia | 14 (34) | |
| Lipodystrophy | 7 (17) | |
| Dilated cardiomyopathy | 3 (7) | |
| Abnormal cognitive-motor development | 1 (2) | |
| Other | 7 (17) | |
IQR – interquartile ranges. CDC – Centers for Disease Control and Prevention. CMV – cytomegalovirus.
In the 24 months prior to transference to adult care, 34% of participants had been diagnosed with AIDS-defining conditions and 22% required hospitalization. Tuberculosis was the leading AIDS-associated disease, followed by herpes zoster and esophageal candidiasis, with incidence rates of 6.1, 3.7 and 2.4 per 100 person-years, respectively.
As described in Table 3, inadequate adherence was reported in 54% of medical records; 12 youths (29%) missed ≥25% appointments, and 24 youths (59%) had delayed ART dispensations from the institutional pharmacy. Altogether, using these different assessments, poor adherence was characterized in 73% of transitioning youths.
Antiretroviral treatment characteristics of 41 HIV infected youths at transition to adult outpatient care in a teaching tertiary care hospital, Sao Paulo, Brazil, 2001 – 2012.
| Variable | n (%) | Median (IQR) |
|---|---|---|
| Time on ART – years | 15 (12 – 17) | |
| Current ART regimen | ||
| None | 2 (5) | |
| 2 NNRTI | 2 (5) | |
| 2 NRTI+NNRTI | 12 (29) | |
| 2 NRTI+PI | 17 (41) | |
| Others | 8 (20) | |
| Current treatment dosing | ||
| q.d. | 2 (5) | |
| b.i.d. | 37 (95) | |
| Number of pills in current ART regimen | 6 (5 – 8) | |
| Number of previous ART regimens | 4 (3 – 6) | |
| Received ART regimen with<3 active drugs | 32 (78) | |
| Underwent HIV genotypic test at pediatric transition period | 12 (29) | |
| ART regimen change due to drug resistance | 10 (83) | |
| Adherence | ||
| Poor adherence reported in medical charts | 22 (54) | |
| ≥25% missed medical appointments | 12 (29) | |
| <85% of timely ART withdrawal | 24 (59) | |
| Any indication of poor adherence* | 30 (73) | |
IQR – interquartile ranges. NRTI – nucleoside and nucleotide reverse transcriptase inhibitors. NNRTI – non-nucleoside reverse transcriptase inhibitors. PI – protease inhibitors. ART – antiretroviral therapy. q.d – once daily. b.i.d – twice daily. *presence of either inadequate adherence in medical records, ≥ 25% missed medical appointments or<85% timely antiretroviral dispensations from the institutional pharmacy.
All cohort participants had long-term ART exposure. Most (78%) had received ART regimens containing less than three drugs early in the course of their treatment. In addition, HIV genotypic tests were performed for 29% of the youths during the last 24 months at pediatric service, triggering modification in ART regimens for 83% of them.
As an important part of youth health, 59% of 34 participants reported being sexually active and four (10%) of them were either pregnant or had partners expecting a child at the moment of transference to adult care service.
DiscussionIn this study, we present demographic and clinical characteristics of a cohort of youths infected with HIV early in childhood (MTCT or blood transfusion) at the transition to adult HIV care as well as HIV VL and CD4+cell count/mm3 at first assessment in the adult care service. Having acquired HIV at the beginning of the Brazilian HIV epidemic, this cohort was exposed to the evolving national recommendations for HIV treatment that guarantees free access to ART through the public health care system (Sistema Unico de Saúde, SUS) since 1992.19–21 As such, they were frequently exposed to regimens currently considered suboptimal, to drugs that are more likely to induce metabolic adverse events or to medications not adapted to children's taste. Moreover, HIV VL tests were not available for clinical monitoring until late in their follow-up.19–21
Poor adherence during the last 24 months at pediatric care was frequent, as in other Brazilian and foreign cohorts of young adults living with HIV since childhood,18,24–29 and discordant of the Swedish single-center cohort, that reports poor adherence by only 12%.28 This highlights the expected difficulties in the clinical management of this special group of patients.
At their first laboratory tests performed in adult care, youths often exhibited uncontrolled disease, as seen by low CD4+cell counts and a high proportion (54%) of patients with detectable HIV VL, similarly to reports from other cohorts of transitioning youths.7,29–31
Dyslipidemia was the most common comorbidity in this population. It could be attributed to metabolic effects of ART and/or to the inflammatory status seen in chronic AIDS, or, alternatively, it could be associated with a less healthy diet and poor exercise habits, as shown previously for this population when still at pediatric care.32,33 Aiming to prevent cardiovascular diseases, our findings highlight the need to prioritize ART drugs with a better metabolic profile provided that they are active to each particular patient. Whenever possible, this should be combined with continuous counseling for healthier diet and exercise. Although lifestyle modifications can be challenging, nutritional counseling and promotion of physical activities by skilled personnel could be important tools in the comprehensive care of this population.
The high frequency of ART modifications after HIV genotyping tests and high number of pills in the current ART regimen support the hypothesis that these youths had a bad ART resistance profile at transition to adult care, as has been previously reported.3,4,6,7,31 In addition, they presented poor immune reconstitution as compared to participants from the IPEC cohort,34 a Brazilian cohort including adults aged 18 and over at the beginning of their HIV treatment. Our cohort of youths had lower median CD4+cell counts compared to the subgroup in IPEC cohort that initiated treatment in the same period (2009 – 2012), i.e. 250 cell/mm3 (IQR 94-460) versus 310 cell/mm3 (IQR 108-551). Furthermore, we observed higher incidence of tuberculosis (6.10/100 person-years) during the two years prior to transference to adult care, as compared to a cohort of 599 adult patients followed at the same institution (1.47/100 person-years).35 Except for higher incidence of tuberculosis, AIDS-associated diseases in our cohort are consistent with findings from the perinatally infected youths in the IMPAACT study,36 in which the most frequently reported morbidities were herpes zoster, oropharyngeal candidiasis, esophageal or pulmonary candidiasis, and pneumonia. Interestingly, despite their low CD4+cell count, youths in our cohort did not present with cerebral toxoplasmosis or Pneumocystis jiroveci pneumonia, commonly seen in adults with impaired immunity and leading causes of AIDS-associated illness in the IPEC cohort.34
Social and household characteristics may add difficulties to the care and wellbeing of these youths. Half of them had lost both parents, 12% were raised in institutions and others were raised by grandparents, who are expected to decrease their support possibilities as they age. As reported by Acree,27 adult health care providers should encourage a slow transition from a social support network to autonomous self-care, avoiding an abrupt break in practical and emotional assistance.
Median attained education of 12 schooling years reflects that most youths complete middle school education, but only half of them reach technical or university-level courses. Our patients had low participation in the workforce (44% employed) and a high proportion (30%) of the cohort was neither employed nor studying. This could reflect the current economic crisis in Brazil, further complicated by frequent absenteeism related to health care appointments.
Most patients in our cohort were sexually active. Sexual activity could be a challenge for these youths. Studies about pregnancy in HIV-infected adolescents and young adults have shown that perinatally infected pregnant youths have higher rates of unintended pregnancies,37 lower adherence to ART,37,38 and lower rates of HIV disclosure when compared to youths infected sexually.39 Thus, one could suggest the adult health care team to be particularly keen to implement an easy channel to talk about sexuality and family planning with these youths.
Accurate evaluation of treatment adherence is a challenge in clinical as well as research settings.40 A strength in our study was the assessment of adherence using a combined evaluation of medical charts, ART dispensation at the institutional pharmacy, and missed appointments with the healthcare providers.
A limited sample size should be considered as a potential weakness of our study, reflecting that MTCT is a relatively rare event in our setting, potentially due to testing and treatment strategies offered to pregnant women in the Brazilian Aids program since 1996. Additionally, the small number or participants in our study also suggests that few HIV-infected children have survived over the first years of the HIV epidemic and reached adult care. Moreover, due to the retrospective nature of the study we failed to collect information on sexual orientation, drug abuse, or psychosocial distress since such information was often lacking or incomplete in medical charts. Finally, the fact that our study addressed patients from a single institution restricts generalizability of our findings. Nevertheless, our study describes one of the first cohorts of perinatally infected youths transitioning to adult care in Latin America, and highlights patient characteristics that are relevant to clinical management in this population. Future multicenter studies with larger sample size and longitudinal follow-up are warranted to better identify predictors of clinical outcomes in this vulnerable population.
In conclusion, most youths living with HIV since early childhood in our Brazilian cohort reached adult outpatient care service with poor adherence to ART, low CD4+cell counts and detectable HIV VL. A third of them had Aids-associated illnesses in the last two years of pediatric follow-up. Based on our results as well as on findings from other cohorts of young adults living with HIV since early childhood, we recommend that adult health care providers should closely monitor these transitioning youths. Comprehensive care should be offered with a multi-professional team, implementing protocols to track treatment failure, stimulate healthier lifestyles, encourage active participation of caregivers in the transition process, afford psychological and social support, and develop a youth-friendly environment prepared for open communication about all aspects of youth's health.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors declare no conflicts of interest
Declaration of interests: none
The authors kindly thank Delsa Nagata, Camila Piccone, Elza Habe and Cleide Maluvayshi for their valuable help with data collection.