To assess the prevalence of hepatitis C virus infection among men who have sex with men (MSM) in Central Brazil, a cross-sectional study was conducted in the City of Goiânia, Central Brazil, using Respondent-Driven Sampling (RDS). All serum samples were tested for anti-HCV and also for alanine aminotransferase (ALT). Anti-HCV positive samples and/or those with elevated ALT were tested for HCV RNA and genotyped. Of the 522 participants, four were found to be anti-HCV positive, and one was also HCV RNA positive (active HCV infection). Elevated ALT was found in 14 individuals. Of these, one showed evidence of acute HCV infection (HCV RNA positive and anti-HCV negative). Therefore, five MSM were positive for either anti-HCV and/or HCV RNA, giving a crude overall HCV prevalence of 1.0%; 1.3% (95% CI: 0.3–5.5) after being weighted by RDSAT. All five individuals reported high-risk sexual behaviors, including two who showed evidence of active HCV infection (genotype 1, subtypes 1a and 1b). Although the study population reported high-risk sexual practices, HCV infection was not more frequent in MSM than in the general Brazilian population.
Hepatitis C virus (HCV) is a major cause of both acute and chronic infection. This infection is a leading cause of cirrhosis, hepatocellular carcinoma, and even liver-related death. However, with the availability of new direct-acting antiviral agents (DAA), cure rates were reported in more than 95% of cases. As a result, hepatitis C is recognized nowadays as a feasible target for elimination, and the World Health Organization encourages countries to develop hepatitis C elimination goals, including the implementation of strategies to expand access to HCV testing, with linkages to treatment, especially among risk populations.1,2
Although HCV is mainly transmitted through the parenteral route, sexually transmitted HCV epidemics among men who have sex with men (MSM) have been reported in Europe, Australia, Asia, and North America, with higher HCV infection rates among human immunodeficiency virus (HIV) infected individuals.3–6 In Brazil, there is currently very little data on HCV infection among MSM.7 Therefore, the aim of this study was to assess the prevalence of HCV infection among MSM living in Central Brazil.
This cross-sectional study was carried out in MSM from Goiânia (a city with approximately 1.5 million inhabitants and, in 2017, the hepatitis C detection rate was 8.3/100,000 inhabitants),8 the capital of the State of Goiás (5.5/100,000 inhabitants),8 located in Central Brazil. From March to November 2014, the respondent-driven sampling (RDS) method was used to recruit MSM.9 The eligibility criteria were: male sex, at least one sexual relation with another man within the past 12 months, age ≥18 years, domiciled in the Goiânia metropolitan area, and presenting a valid study recruitment coupon. Among the 530 MSM who presented a valid study recruitment coupon, eight were excluded (six were under 18 years old and two refused to give a blood sample).
After written informed consent was obtained from all participants, they were interviewed to collect information about the size of their personal network, relationship with the recruiter, and sociodemographic and behavioral characteristics. This study was approved by the Ethics Committee of the Federal University of Goiás (reference number 042/13).
All serum samples were tested using a fourth-generation enzyme-linked immunosorbent assay (ELISA) for the presence of antibodies against HCV (anti-HCV; Biokit Anti-HCV Bioelisa, Barcelona, Spain). In addition, serum levels of alanine aminotransferase (ALT) were determined. Baseline serum samples were also tested by a fourth-generation ELISA for the presence of anti-HIV-1.2.0 (Murex Biotech, Dartford, UK).
Anti-HCV positive samples and/or those which had elevated ALT (>40 IU/L) were submitted to HCV RNA detection by reverse transcription (RT) nested polymerase chain reaction (PCR) with primers complementary to the conserved area of the 5′ non-coding region of HCV, as described previously.10 Positive samples were genotyped using a line probe assay (Inno-LiPA HCV II, Innogenetics, Ghent, Belgium).
Prevalence and 95% confidence intervals (CI) were estimated using RDS Analysis Tool (RDSAT) v.5.6 (http://rds-analysis-tool.software.informer.com/versions/). To reduce possible biases associated with chain referral sampling, RDSAT provides weights for each participant based on his social network size and recruitment patterns.11
A total of 522 MSM were eligible and accepted to participate in the study. Most were younger than 25 years (60%), self-identified as gay (74.9%), self-reported skin color brown or mixed (pardo) (59%), single (76.9%), and had attended high school (10–12 years of study, 63.9%). Only a few MSM had received a blood transfusion (5.3%). One third of the participants reported use of illicit drugs (35.1%). Only 1.3% of MSM reported injection drug use (IDU). Regarding sexual behaviors, the majority of MSM had more than 10 lifetime sexual partners (59.7%) and reported both receptive/insertive anal intercourse (65.1%), and illicit drug or alcohol use during sex (61.5%). Half of the individuals also reported high-risk practices such as sex with drug users (52.2%), group sex (45.1%), and unprotected anal intercourse (44.3%). One third of the study population reported rectal trauma with bleeding (34.2%), sex for money (33.1%), and history of sexually transmitted infections (STI, 30.5%).
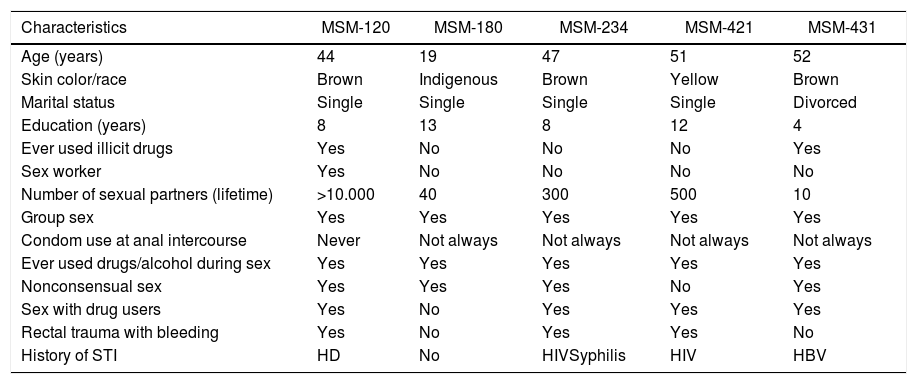
Of the 522 MSM, four were found to be anti-HCV positive, and one was also HCV RNA positive, revealing evidence of active HCV infection. Elevated ALT was found in 14 individuals. Of these, one showed evidence of acute HCV infection (HCV RNA positive and anti-HCV negative). Therefore, five participants were positive for either anti-HCV and/or HCV RNA, giving a crude overall HCV prevalence of 1.0%; 1.3% (95% CI: 0.3–5.5) after being weighted by RDSAT. All five individuals reported high-risk sexual behaviors (Table 1), including the two MSM that showed evidence of active HCV infection: MSM-180 (infected with genotype 1/subtype 1b) and MSM-421 (HIV-positive and chronic infection with genotype 1/subtype 1a), as well as three study participants who had cleared their infection (positive for HCV antibodies but negative for HCV RNA), of whom one was a sex worker (MSM-120), and two reported history of HIV infection and syphilis (MSM-234), or hepatitis B (MSM-431).
Sociodemographic and risk behavior characteristics of the five HCV infected MSM, Goiânia-GO, 2014.
| Characteristics | MSM-120 | MSM-180 | MSM-234 | MSM-421 | MSM-431 |
|---|---|---|---|---|---|
| Age (years) | 44 | 19 | 47 | 51 | 52 |
| Skin color/race | Brown | Indigenous | Brown | Yellow | Brown |
| Marital status | Single | Single | Single | Single | Divorced |
| Education (years) | 8 | 13 | 8 | 12 | 4 |
| Ever used illicit drugs | Yes | No | No | No | Yes |
| Sex worker | Yes | No | No | No | No |
| Number of sexual partners (lifetime) | >10.000 | 40 | 300 | 500 | 10 |
| Group sex | Yes | Yes | Yes | Yes | Yes |
| Condom use at anal intercourse | Never | Not always | Not always | Not always | Not always |
| Ever used drugs/alcohol during sex | Yes | Yes | Yes | Yes | Yes |
| Nonconsensual sex | Yes | Yes | Yes | No | Yes |
| Sex with drug users | Yes | No | Yes | Yes | Yes |
| Rectal trauma with bleeding | Yes | No | Yes | Yes | No |
| History of STI | HD | No | HIVSyphilis | HIV | HBV |
HBV, hepatitis B virus; HCV, hepatitis C virus; HD, Haemophilus ducreyi; HIV, human immunodeficiency virus; MSM, men who have sex with men; STI, sexually transmitted infections.
This study is the second concerning HCV infection in MSM in Brazil. The prevalence of HCV infection found in this study was similar to that determined in another study among Brazilian MSM (City of Campinas, State of São Paulo: 1.2%; 1.0%; 95% CI: 0.0–3.2 after being weighted by RDSAT).7 This prevalence was also comparable to that obtained in a multicentric population-based study conducted from 2005 to 2009 in the capital cities of Brazil (1.38%; 95% CI: 1.12–1.64).12 In 2016, the anti-HCV prevalence in Brazil was estimated to be 0.7%.8
A wide variation in HCV prevalence rates among MSM has been found worldwide, with higher rates among MSM who reported IDU compared to those who did not.13,14 Further, HCV infection is more frequently found among HIV-infected MSM than in HIV-negative MSM.3,5,13 In line with these studies, we found a low prevalence of HCV among MSM in Central Brazil. In fact, of the 522 participants, most (80%) were HIV-negative and only 1.3% reported IDU (two of seven shared syringes/needles, and one was HCV-positive).
More recently, however, an increasing burden of HIV has been found in the MSM population in Brazil,15 pointing towards a possible increase in the prevalence of HCV infection. Therefore, studies with a large sample of HIV-infected MSM are needed to monitor trends of HCV infection in this population. In addition, an increase in sexually acquired HCV among HIV-negative MSM using preexposure prophylaxis (PrEP) has been reported elsewhere.16 Further studies are also necessary to investigate whether the introduction of PrEP (December 2017) in Brazil will change the epidemiology of hepatitis C among HIV-negative MSM.
FundingThis study was funded by the Brazilian Ministry of Health/Secretariat of Health Surveillance/Department of STD, Aids and Viral Hepatitis through the Project of International Technical Cooperation BRAK57 between the Brazilian Government and the United Nations Office on Drugs and Crime-UNODC.
Conflict of interestThe authors have no conflicts of interest to declare.
We would like to thank all MSM that participated in this study and the collaborators of governmental and non-governmental organizations of LGBT (Lesbian, Gay, Bisexual and Transgender) movement, and Brian Ream edited this manuscript in English.