Antiretroviral therapy (ART) has decreased AIDS incidence and mortality, rendering comorbidities, such as hepatitis B more relevant for people living with human immunodeficiency virus (HIV). Since antiretroviral drugs may also inhibit hepatitis B virus (HBV) replication, analyzing the impact of ART on management of hepatitis B in this population is important.
ObjectiveTo assess HBV viremia among HIV/HBV coinfected individuals on ART and its associated factors.
MethodFor this cross-sectional study, HIV/HBV-coinfected individuals, aged over 18 years, who were on ART for over six months and receiving care at an outpatient clinic in São Paulo were recruited. Sociodemographic characteristics, information about viral exposure, clinical and laboratory data, including evaluation of liver fibrosis were obtained. Plasma HBV DNA was measured by polymerase chain reaction. Viral genome sequencing was conducted for genotyping and identification of drug resistance-conferring mutations if viral load exceeded 900 IU/mL.
ResultsOut of 2,946 patients who attended the clinic in 2015, 83 were eligible and 56 evaluated. Plasma HBV DNA was detected in 16 (28.6%) (95% CI: 18.0–41.3%), all on lamivudine and tenofovir treatment. HBV DNA detection was associated with lower education (p = 0.015), higher international normalized ratios (p = 0.045), history of an AIDS-defining illness [OR: 3.43 (95% CI: 1.10–11.50)], and HBeAg detection [OR: 6.60 (95% CI: 1.84–23.6)]. In contrast, a last CD4+ count above 500 cells/mm3 in the year prior to inclusion [OR: 0.18 (95% CI: 0.04–0.71)] and detection of anti-HBe [OR: 0.21 (95% CI: 0.04–0.99)] were negatively associated. Patients with HBV DNA above 900 IU/mL were infected with subgenotypes A1 (n = 3) and D2 (n = 1), and exhibited viral mutations associated with total resistance to lamivudine and partial resistance to entecavir.
ConclusionsDespite being on ART, a significant proportion of HIV/HBV-coinfected individuals present HBV viremia. Characterization of factors that are associated with this finding may help professionals provide better management to these patients.
Hepatitis B virus (HBV) infection is the most common chronic viral infection in the world, constituting an important public health problem.1 Globally, it is estimated that 257 million people are living with the infection. However, prevalence rates vary by geographical region. Hepatitis B resulted in 887,000 deaths in 2015 mainly caused by complications such as cirrhosis and hepatocellular carcinoma. Vaccination is an effective way of preventing HBV infection; however, most of the chronic carriers of this infection were born before this immunization became available.2 Since HBV and HIV share common modes of transmission, including unprotected sex and use of injectable drugs, HIV/HBV coinfections are likely to occur.3,4 In fact, the World Health Organization (WHO) estimated that 36.7 million people were living with HIV worldwide in 2015, of whom 2.7 million were HBV-coinfected.2 Likewise, HIV/HBV coinfection was identified in 5.2% of hepatitis B reported cases in Brazil from 2007 to 2017.5
In a context in which antiretroviral therapy (ART) has significantly decreased AIDS incidence and mortality, comorbidities such as hepatitis B have become more relevant for the comprehensive care of people living with HIV (PLH).4 Current Brazilian,6 American7,8 and European9,10 guidelines recommend initiating ART for all patients with HIV infection, even if they are asymptomatic and regardless of immune status as assessed by CD4+ cell count. For those coinfected with HBV, however, antiretroviral regimens that include tenofovir disoproxil fumarate (TDF), tenofovir alafenamide (TAF), TDF/lamivudine (3TC) or TDF/emtricitabine combinations are considered preferable. In Brazil, national guidelines recommend HIV/HBV coinfected patients should be receive with tenofovir as part of their regimen. Despite being regarded as the ideal antiviral to be used in these cases, some patients taking tenofovir may yet exhibit incomplete HBV suppression for unclear reasons, yielding persistent or transient hepatitis B viremia.11
Analyzing the impact of ART on hepatitis B in this coinfected population during clinical follow-up is thus important to help guide proper clinical management. This study aimed to assess plasma HBV DNA among HIV/HBV coinfected individuals on ART in outpatient care in São Paulo, Brazil, and to identify factors associated with HBV viremia. Furthermore, viral genomic sequencing was used to detect drug resistance-conferring mutations among hepatitis B viremic patients.
Materials and methodsStudy design and selection of patientsThis cross-sectional was carried out at the HIV/Aids Clinic of the Division of Infectious and Parasitic Diseases of the Hospital das Clínicas, affiliated to the Universidade de São Paulo Medical School (SEAP HIV/AIDS - HCFMUSP), where interdisciplinary and comprehensive care is provided to adults living with HIV.
Medical charts of patients who attended the clinic in 2015 were reviewed in search for eligible subjects: patients aged over 18 years, diagnosed with HIV infection and chronic HBV (HBsAg positivity in serum for six months or more), and using antiretrovirals for at least six months. There was no exclusion criterion.
Data collectionPatients who met inclusion criteria were invited to participate in the study. Information regarding variables of interest was collected through individual interviews, chart analysis, evaluation of laboratory tests and transient hepatic elastography, and data recorded in a standardized form, specifically elaborated for the study.
Study outcomes included:
- -
Presence of HBV viremia, measured by plasma detection of viral deoxyribonucleic acid (DNA);
- -
HBV genotype (if viremia above 900 IU/mL);
- -
Presence of drug resistance-conferring mutations identified by sequencing the RT region of the HBV genome (if viremia above 900 IU/mL).
HBV viremia was assessed by testing plasma samples with quantitative real-time polymerase chain reaction (Abbott Real-time HBV®), following manufacturers’ instructions.12 Using a 0.5 mL sample preparation, HBV DNA detection threshold of this test is 6.4 IU/mL (95% CI: 3.97–13.03 IU/mL). However, for viral load quantification a lower limit of 10 IU/mL was used.
Samples that exhibited HBV DNA concentrations above 900 IU/mL were then submitted to sequencing of the RT region of the HBV genome in search of drug resistance-conferring mutations, as previously reported.13 An HBV DNA threshold of 900 IU/mL was used due to the test sensitivity.
Independent variables included:
- -
Sociodemographic characteristics and anthropometric measures: sex, age, self-reported ethnicity, schooling, and body mass index (BMI);
- -
Exposure to HBV and/or HIV: time since diagnosis of HBV and HIV infections, exposure categories, history of imprisonment, and other sexually transmitted infections (STI);
- -
Alcohol consumption (assessed by a modified AUDIT questionnaire14);
- -
Clinical data: history of AIDS-defining illnesses,15 ART (prescribed antiviral regimens, duration of use, self-reported adherence to ART assessed by a questionnaire modified from the Medication Adherence Self-Report Inventory – MASRI16), and use of other anti-HBV drugs;
- -
Laboratory data: serum HBeAg and anti-HBe, last CD4+ count in the year before inclusion, nadir CD4+ count, last HIV viral load in the six months prior to inclusion, platelet count, international normalized ratio (INR), serum transaminases, total bilirubin, gamma glutamyl transferase, total protein, albumin, gamma globulin, alpha-fetoprotein, serum anti-HCV antibodies, and plasma HCV RNA;
- -
Transient liver elastography findings: liver stiffness and controlled attenuation parameter (CAP).
Data were entered into a Microsoft Excel 2010 spreadsheet and analyzed with the aid of the statistical program IBM SPSS Statistics for Windows, Version 23.0, Armonk, NY.
For descriptive statistics, categorical variables of interest were presented in frequency tables, and quantitative variables with central tendency measures and variability.
Factors associated with HBV viremia were then sought after by correlating HBV DNA detection with patients’ socioeconomic and anthropometric characteristics, and variables related to HIV and HBV exposure, clinical features, as well as with results of laboratory tests and imaging exams. Fisher’s exact test or the chi-square test with calculation of odds ratios (OR) and their respective 95% confidence intervals (95% CI) was used to compare HBV viremic and nonviremic individuals. Quantitative variables were assessed with the nonparametric Mann–Whitney test assessment of variable distribution by the Shapiro–Wilk test. A statistical significance level of 5% was adopted for all analyses.
Ethical issuesData collection was initiated only after the study protocol was approved by Institutional Review Boards. Study participation was voluntary and followed informed consent. Confidentiality and anonymity of participants were assured throughout the investigation.
ResultsFrom January 1 to December 31, 2015, 2,946 PLH attended medical follow-up consultations at SEAP HIV/AIDS - HCFMUSP. Of these, 83 exhibited serum HBsAg for at least six months, yielding an overall prevalence of 2.8% of PLH, who were chronically coinfected with HBV (95% CI: 2.3–3.4%). They were all receiving ART for six months or longer and were, therefore, eligible for the study. However, 27 of them could not be evaluated due to refusal (n = 11), death (n = 4), loss to follow-up (n = 4), incorrect contact information (n = 5), referral to other HIV clinics (n = 2), or HBsAg seroreversion (n = 1). Therefore, the study cohort consisted of 56 HIV/HBV coinfected adults. These individuals who could not be evaluated were not significantly different in terms of sex [96% male (95% CI: 89–99%) versus 93% (95% CI: 78–98%)] and median age [53 years (95% CI: 51–55) versus 50 (95% CI: 46–54)].
In the remaining cohort, most participants were male (n = 54, 96%), aged over 50 (n = 37, 66%), white (n = 33, 59%), reported at least eight years of schooling (n = 34, 61%), and presented with normal weight (n = 36, 64%). The median time since HIV diagnosis was 18.1 years (2.0–28.3 years), and the median time since HBV diagnosis was 15.0 years (1.0–29.0 years). Concerning exposure to viral infections, 42 participants reported being men who have sex with men, and three had been in prison (for 15 days, 12 months and 44 months). A total of 41 (73%) individuals had an informed history of at least one episode of STI [syphilis (n = 31), gonorrhea (n = 21), genital warts (n = 10) and herpes (n = 2)]. Seventeen (30%) patients reported more than one STI. History of injectable drug use and of blood transfusion was reported by three patients each.
Screening for hepatitis C disclosed 10 (18%) participants with evidence of previous HCV infection (positive EIA test, but HCV RNA PCR negative) and three (5%) had evidence of HCV viremia before inclusion in the study. All HCV-coinfected patients had been treated with pegylated interferon for six months (n = 1) or 12 months (n = 2), 12, 8 and 11 years, respectively, before inclusion in the study and exhibited sustained virologic responses.
As for alcohol consumption, 44 (79%) reported none or drinking once a month or less often; in contrast, 13 (28%) informed at least one episode of drinking six or more shots in a single occasion.
In terms of HIV disease, 23 (41%) reported having experienced an AIDS-defining illness, most often pulmonary tuberculosis (n = 11), Pneumocystis jirovecii pneumonia (n = 5), CMV disease [retinitis or in other organs but the liver, spleen or lymph nodes (n = 4)], or Kaposi’s sarcoma (n = 4). Nine (16%) participants had two or more illnesses.
As far as anti-HBV medication is concerned, 54 (96%) patients in our cohort were taking lamivudine and tenofovir at the time of inclusion in the study. Two participants, in contrast, were not receiving tenofovir at baseline due to previous renal toxicity, experienced after 104 and 137 months of use of this drug. At inclusion in our study one of these patients was receiving lamivudine plus entecavir, whereas the other was using lamivudine alone. As for other anti-HBV medications, three (5%) individuals reported having used pegylated interferon to treat chronic hepatitis C, as previously mentioned, and two (4%) patients received entecavir before inclusion in the study, while one was using entecavir at the time of inclusion in combination with ART regimens that included lamivudine but not tenofovir.
Additionally, 31 (55%) patients were on ART regimens that included non-nucleoside reverse transcriptase inhibitor drugs, 24 (43%) were on protease inhibitors, five (9%) were taking integrase inhibitors and one was receiving enfuvirtide. Most patients reported good adherence to ART: 51 (91%) informed having taken more than 85% of prescribed pills in the previous month.
Plasma detection of HBV DNA was achieved in 16 individuals in our cohort (28.6%, 95% CI: 18.0–41.3%), and in 12 (96%) HBV viral load was less than 900 IU/mL.
In univariate analysis to identify factors associated with HBV viremia, international normalized ratios (INR) was significantly higher (p = 0.045) among viremic patients (Table 1) compared with nonviremic HIV/HBV coinfected individuals. In contrast, no significant difference was shown between the two groups regarding age, BMI, time since HIV and HBV diagnoses, platelet count, serum ALT, AST, bilirubin, gamma glutamyl transferase, total protein, albumin, gamma globulin, and alpha-fetoprotein concentrations.
Quantitative variables according to HBV viremia.
| HBV viremia | |||||
|---|---|---|---|---|---|
| Variables | No | Yes | p-value | ||
| Median | IQR | Median | IQR | ||
| Sociodemographic characteristics and anthropometric measures | |||||
| Age (years) | 52.0 | 48.0–57.0 | 55.0 | 49.0–59.0 | 0.643 |
| BMI (kg/m2) | 23.8 | 22.0–25.6 | 24.8 | 20.7–27.0 | 0.663 |
| Time since diagnosis (years) | |||||
| HIV | 17.9 | 15.7–20.9 | 19.8 | 15.0–22.0 | 0.828 |
| HBV | 15.0 | 10.5–18.0 | 12.5 | 3.5–16.5 | 0.167 |
| Time using ART (years) | |||||
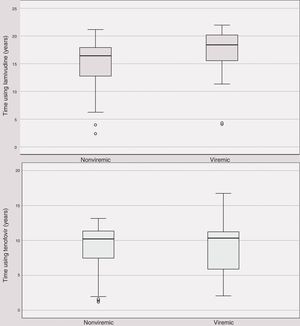
| Lamivudine | 16.0 | 13.0–18.0 | 18.0 | 16.0–20.0 | 0.130 |
| Tenofovir | 10.2 | 7.5–11.4 | 10.3 | 5.9–11.2 | 0.779 |
| Laboratory data | |||||
| Platelet count (x 103/mm3) | 193.5 | 163.0–232.5 | 181.0 | 163.0–232.5 | 0.663 |
| ALT (IU/L) | 25.0 | 19.0–39.0 | 31.0 | 20.0–51.0 | 0.268 |
| AST (IU/L) | 24.0 | 19.0–36.0 | 27.0 | 22.0–33.0 | 0.280 |
| Total bilirubin (mg/dL) | 0.40 | 0.29–0.70 | 0.60 | 0.41–1.02 | 0.102 |
| GGT (IU/L) | 34.0 | 22.0–79.0 | 44.0 | 23.0–81.0 | 0.580 |
| INR | 1.00 | 0.97–1.07 | 1.06 | 1.03–1.10 | 0.045 |
| Total protein (g/dL) | 7.5 | 7.2–7.8 | 7.6 | 7.3–8.3 | 0.501 |
| Albumin (g/dL) | 4.1 | 3.9–4.3 | 4.0 | 3.8–4.1 | 0.065 |
| Gamma globulin (g/dL) | 1.3 | 1.1–1.6 | 1.6 | 1.3–1.8 | 0.147 |
| Alpha-fetoprotein (ng/mL) | 2.5 | 2.1–3.3 | 2.6 | 2.2–3.7 | 0.771 |
HBV, hepatitis B virus; IQR, interquartile range; BMI, body mass index; HIV, human immunodeficiency virus; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyl transferase; INR, international normalized ratio.
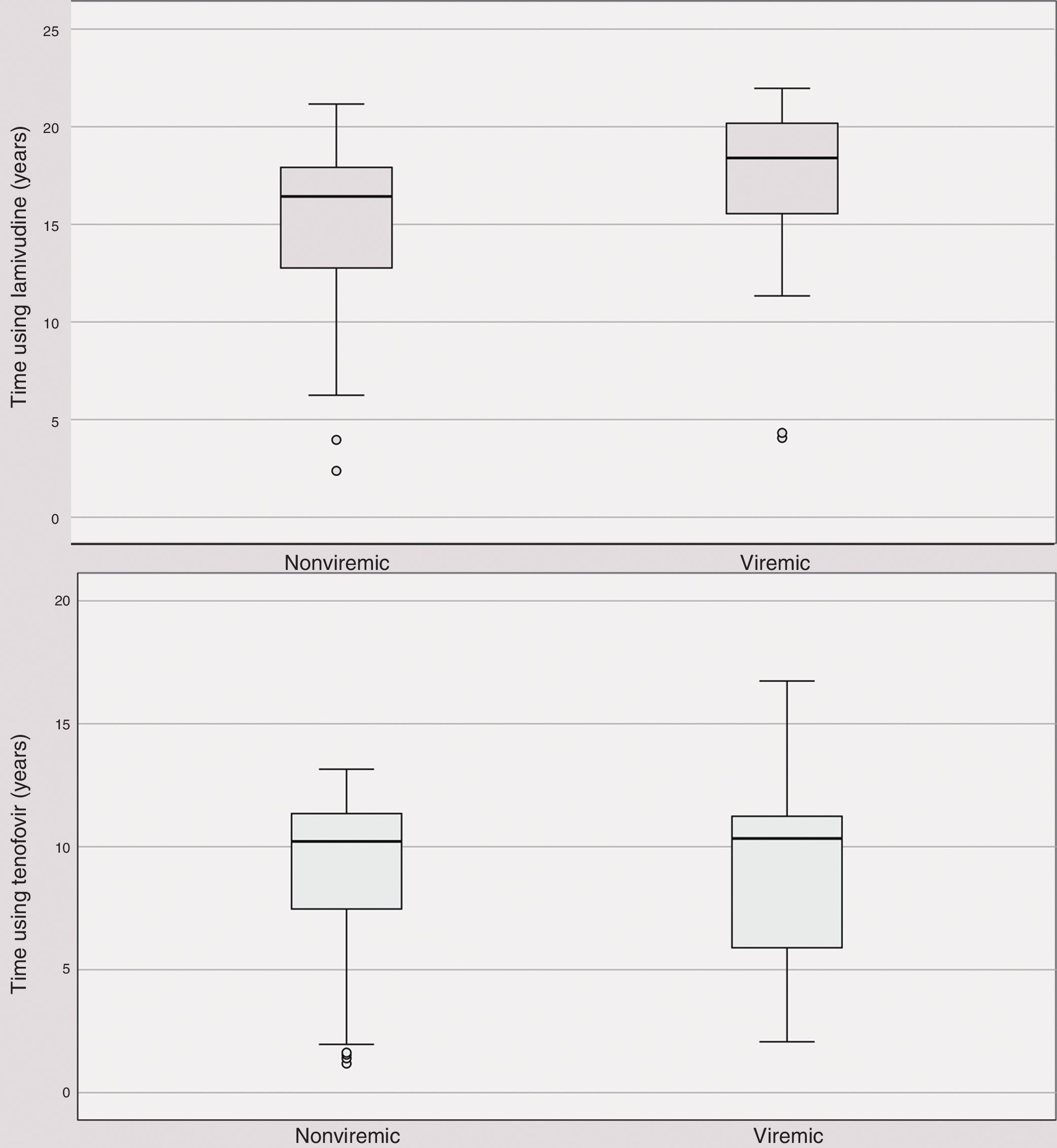
Time using anti-HBV medication did not differ between HBV viremic and nonviremic patients (Fig. 1A and 1B).
A comparison of categorical variables between HBV viremic and nonviremic patients is shown in Table 2. HBV viremia was positively associated with lower levels of schooling (0% viremic among individuals with university degree versus 50% viremic among those with no education or incomplete basic education (p = 0.015), history of an AIDS-defining illness [OR: 3.43 (95% CI: 1.10–11.50); p = 0.040], and serum HBeAg detection [OR: 6.60 (95% CI: 1.84–23.6); p = 0.003]. In contrast, last CD4+ count over 500 cells/mm3 in the year before inclusion [OR: 0.18 (95% CI: 0.04–0.71); p = 0.016] and serum anti-HBe detection [OR: 0.21 (95% CI: 0.04–0.99); p = 0.043] were negatively associated with HBV viremia.
Categorical variables according to HBV viremia.
| HBV viremia | |||||||
|---|---|---|---|---|---|---|---|
| Variables | No (N = 40) | Yes (N = 16) | OR | 95% CI | p-value | ||
| N | % | N | % | ||||
| Sex | 0.506 | ||||||
| Male | 38 | 70 | 16 | 30 | 1 | – | |
| Female | 2 | 100 | 0 | 0 | 0 | 0 | |
| Ethnicity | 0.123 | ||||||
| White | 26 | 79 | 7 | 21 | 1 | – | |
| Non-White | 14 | 61 | 9 | 39 | 2.30 | 0.73–7.70 | |
| Schooling | 0.015a | ||||||
| None or incomplete basic education | 6 | 50 | 6 | 50 | 1 | – | |
| Basic education | 7 | 70 | 3 | 30 | 0.42 | 0.07–2.49 | |
| Medium education | 15 | 68 | 7 | 32 | 0.46 | 0.10–2.10 | |
| University degree | 12 | 100 | 0 | 0 | 0 | 0 | |
| HIV exposure categories | 0.111 | ||||||
| Heterosexual | 10 | 91 | 1 | 9 | 1 | – | – |
| MSM | 26 | 67 | 13 | 33 | 5.00 | 0.89–11.90 | |
| IDU | 1 | 33 | 2 | 67 | 20.00 | 0.12–68.00 | |
| Blood transfusion | 3 | 100 | 0 | 0 | 0 | 0 | |
| Alcohol consumption | 0.269 | ||||||
| Never | 20 | 80 | 5 | 20 | 1 | – | |
| ≤ Once/month | 12 | 63 | 7 | 37 | 2.33 | 0.60–9.02 | |
| ≥Twice/month | 8 | 67 | 4 | 33 | 2.00 | 0.40–9.40 | |
| AIDS-defining illness | 0.040 | ||||||
| No | 27 | 82 | 6 | 18 | 1 | – | |
| Yes | 13 | 57 | 10 | 43 | 3.43 | 1.10–11.50 | |
| Last CD4+ count (cells/mm3) | 0.016a | ||||||
| ≤500 | 5 | 42 | 7 | 58 | 1 | – | |
| >500 | 35 | 80 | 9 | 20 | 0.18 | 0.04–0.71 | |
| Nadir CD4+ count (cells/mm3) | 0.660a | ||||||
| <100 | 14 | 64 | 8 | 36 | 1 | – | |
| 100–500 | 19 | 76 | 6 | 24 | 0.55 | 0.15–1.55 | |
| >500 | 7 | 78 | 2 | 22 | 0.50 | 0.08–3.00 | |
| HIV viral load | 0.135 | ||||||
| Undetectable | 38 | 75 | 13 | 25 | 1 | – | |
| Detectable | 2 | 40 | 3 | 60 | 4.38 | 0.65–29.20 | |
| HBeAg | 0.003 | ||||||
| Nonreactive | 30 | 86 | 5 | 14 | 1 | – | |
| Reactive | 10 | 48 | 11 | 52 | 6.60 | 1.84–23.60 | |
| Anti-HBe | 0.043 | ||||||
| Nonreactive | 24 | 63 | 14 | 37 | 1 | – | |
| Reactive | 16 | 89 | 2 | 11 | 0.21 | 0.04–0.99 | |
| Anti-HCV | 0.301 | ||||||
| Nonreactive | 34 | 74 | 12 | 26 | 1 | – | |
| Reactive | 6 | 60 | 4 | 40 | 1.89 | 0.45–7.89 | |
| HCV RNAb | |||||||
| Undetectable | 4 | 57 | 3 | 43 | 1 | – | 0.666 |
| Detectable | 2 | 67 | 1 | 33 | 0.66 | 0.03–11.28 | |
| Liver stiffness (kPa)c | 0.223 | ||||||
| <7.2 | 19 | 76 | 6 | 24 | 1 | – | |
| 7.2–11.0 | 8 | 80 | 2 | 20 | 0.79 | 0.13–4.70 | |
| >11.0 | 3 | 50 | 3 | 50 | 3.16 | 0.50–20.00 | |
| CAP (dB/m)c | 0.398 | ||||||
| <215 | 12 | 80 | 3 | 20 | 1 | – | |
| 215–300 | 15 | 68 | 7 | 32 | 1.80 | 0.39–8.80 | |
| >300 | 3 | 75 | 1 | 25 | 1.33 | 0.09–17.80 | |
HBV, hepatitis B virus; OR, odds ratio; 95% CI, 95% confidence interval; HIV, human immunodeficiency virus; MSM, men who have sex with men; IDU, injection drug use; AIDS, acquired immunodeficiency syndrome; HBeAg, hepatitis B e-antigen; Anti-HBe, hepatitis B e-antibody; Anti-HCV, hepatitis C antibody; HCV RNA, hepatitis C virus RNA; CAP, controlled attenuation parameter.
Furthermore, age, ethnicity, HIV exposure category, alcohol consumption, nadir CD4+ count, HIV viral load, anti-HCV, and HCV RNA detection were not associated with HBV viremia. Likewise, transient liver elastography findings (liver stiffness and CAP) did not differ according to HBV viremia.
Out of four patients who exhibited HBV viral loads above 900 IU/mL, three were infected with HBV subgenotype A1 and the fourth with subgenotype D2. In all of them, drug resistance-conferring mutations associated with total resistance to lamivudine and with partial resistance to entecavir (Table 3) were demonstrated.
HBV viral load, HBV subgenotypes, viral mutations, drug resistance profile, and time using lamivudine and tenofovir in patients who presented HBV viral load greater than 900 IU/mL.
| Patient | HBV viral load (IU/mL) | HBV subgenotype | RT region mutations | Drug resistance profile | Time using lamivudine (years) | Time using tenofovir (years) | |||
|---|---|---|---|---|---|---|---|---|---|
| LAM | ADV | ETV | TDF | ||||||
| 1 | 929 | A1 | rtL180 M + rtM204 V + rtL229F | R | S | I | S | 19.5 | 11.1 |
| 2 | 2,877 | D2 | rtV173 L + rtL180 M + rtM204V | R | S | I | S | 19.2 | 11.1 |
| 3 | 49,425,077 | A1 | rtL180 M + rtA200 V + rtM204I | R | S | I | S | 20.9 | 7.1 |
| 4 | 2,518 | A1 | rtL180 M + rtM204 V + rtL229 V + rtV253I | R | S | I | S | 17.6 | 2.1 |
HBV, hepatitis B virus; LAM, lamivudine; ADV, adefovir; ETV, entecavir; TDF, tenofovir disoproxil fumarate; S, sensitive; I, intermediate susceptibility; R, resistant.
Furthermore, positions rt106, rt126, rt134, and rt269 were also analyzed. In individuals infected with HBV subgenotype A1, the following amino acid patterns were identified at these positions: rtS106, rtY126, rtD134, and rtI269, whereas in the individual infected with subgenotype D2, we found: rtS106, rtR126, rtD134 and rtI269.
DiscussionIn our cohort, followed up at an University outpatient clinic in São Paulo, 83 (2.8%) of patients were identified as HIV/HBV-coinfected (95% CI: 2.3–3.4%), and among them, 56/83 (28.6%, 95% CI: 18.0–41.3%) presented HBV viremia despite being treated with anti-HBV antivirals.
It is important to highlight that participants in our cohort were predominantly men who had sex with men, in accordance with findings of previous studies carried out in the same clinic.17,18 As far as education was concerned, 61% reported at least 11 schooling years, a feature of the clinic clientele, as previously reported.19
Nevertheless, the rate of HBV viremic individuals - 28.6% - was high, despite long-term use of antivirals that are active against the virus (median time 10.3 years for tenofovir and 18.0 years for lamivudine). Tenofovir and lamivudine were used by all viremic patients. In addition, patients not taking tenofovir due to past renal toxicity had no HBV viremia.
HBV suppression in HIV/HBV-coinfected individuals on ART has been previously investigated in longitudinal studies in several countries, and usually a more favorable response was seen compared to our findings. de Vries-Sluijs et al.,20 for instance, in a multicenter study with median follow-up of 55 months, showed that among HBeAg-positive individuals, the cumulative probability of achieving virologic response after 1, 2, 3, 4 and 5 years of treatment was 31%, 70%, 83%, 88% and 92%, respectively. Among HBeAg-negative patients, the virologic response in the first four years of therapy was 47%, 85%, 85% and 100%, respectively. Likewise, Price et al.21 reported HBV suppression in more than 85% of cases after three years of tenofovir use. Studying patients from Australia, United States and Thailand, Matthews et al.22 detected HBV viremia in 20.8%, and the outcome was associated with serum HBeAg detection, HIV viral load, CD4+ cell count lower than 200 cells/mm3, use of ART for less than two years, self-reported adherence to therapy below 95%, and HBV monotherapy with lamivudine/emtricitabine or TDF. More recently, Huang et al.23 highlighted that the use of tenofovir-containing regimens as initial antiviral therapy is independently associated with HBV suppression.
Lower education was associated with HBV viremia in our cohort. One may hypothesize that this association may be a consequence of lower adherence to therapy, as previously proposed by Carvalho et al.24 In this cohort patients with university degrees were more adherent to therapy compared to those with medium-level education or less (OR: 7.0; p = 0.037). From a broader perspective, Tavares et al.25 pointed out that among Brazilian patients with other chronic diseases, lower education was also associated with poorer adherence to therapy, highlighting the need to take this into consideration in a comprehensive care approach. However, we were not able to show a correlation between education and adherence to therapy in our study. As 95% of cohort participants reported having taken more than 85% of prescribed pills in the 30 days prior to inclusion, adherence to treatment could not be included in the analysis.
Assessing adherence to therapy by means of patients’ self-report should also be put in context since limitations of this technique have been discussed in a previous study conducted at the same clinic. Among those self-reporting good adherence to ART, Gutierrez et al.26 evaluated drug dispensation registries and found that only 39.3%, 54.1%, and 69.3% of patients had in fact collected greater than or equal to 95%, 90% and 80% of prescribed pills in the previous year, respectively. In a systematic review, Lieveld et al.27 reported that mean adherence to hepatitis B therapy ranges from 81 to 99%, and full adherence from 66 to 92% of patients in different studies. Higher adherence rates are found by patients’ self-report, whereas surveys that employed pill counts usually yield poorer rates. However, these investigators emphasize that so far there is no consensus on what adherence rate to HBV therapy should be regarded as optimal. We believe that assessment by patients’ self-report might have overestimated adherence to therapy in our study.
Although HIV viral load was not significantly associated with HBV viremia in our cohort, we should highlight that 60% of those with detectable HIV RNA in the last test performed 180 days before inclusion also exhibited HBV viremia. The patient who presented the highest hepatitis B viral load in our cohort (49,425,077 IU/mL) reported having discontinued ART in the month prior to inclusion in the study because of a surgical procedure. As a matter of fact, his HIV viral load was 3,297 RNA copies/mL on the same date. In addition, two other individuals exhibited simultaneous HIV and HBV viremia. Of these, one exhibited an HBV DNA concentration of 929 IU/mL, whereas the other had an HBV load below quantitation threshold (less than 10 IU/mL). The limited number of HIV viremic patients in our cohort may have reduced the statistical power to demonstrate association of HIV viremia with the study outcome.
In a cohort study of HIV/HBV-coinfected individuals in the United States,28 it has been shown that in patients diagnosed with HBV infection after diagnosis of HIV infection, combined antiretroviral therapy was associated with a reduction in the risk of chronic HBV infection (OR: 0.18; 95% CI: 0.04–0.79). Similarly, in the present study, history of AIDS-defining diseases resulted in an increased risk of HBV DNA detection (OR: 3.43; p = 0.040). Conversely, a CD4+ count above 500 cells/mm3 in the 365 days prior to inclusion was protective of the outcome (OR: 0.18; p = 0.016).
Another factor directly associated with detection of plasma HBV DNA in our study was the detection of the viral e antigen (HBeAg). In contrast, anti-HBe detection was shown to be protective of the outcome. The association of both variables with the main study outcome was expected, given that HBeAg is a recognized biomarker of HBV replication, whereas anti-HBe seroconversion corresponds to suppression of replication. The same finding had previously been described in coinfected patients from the same clinic, by Mendes-Correa et al.29
A direct association of higher values of INR with the detection of HBV DNA was also verified in this study, suggesting the possibility of a relationship between the outcome and more advanced liver disease. Nonetheless, there was no association of HBV viremia and significant alterations of other biomarkers, such as aminotransferases, total bilirubin, gamma globulins and albumin, has been observed, nor was it related to hepatic rigidity. Although we had no baseline data of the studied individuals, due to the study cross-sectional design, it is well known that nucleos(t)ide analogue therapy can reverse fibrosis, in addition to improving liver function in a substantial number of patients.30,31
In patients from whom it was technically feasible to perform HBV DNA sequencing, a predominance of individuals with the A1 subgenotype was detected, followed by the D2 subgenotype, which agrees with other Brazilian studies.32–34 This observation suggests an effect of “Afrodescendence” in addition to the influence of European colonization in our country. Soriano et al.35 using EuroSIDA study data demonstrated that most patients with genotype A infection on that continent were men who had sex with men, while injecting drug users had genotype D. Moreover, Boyd et al.11 identified genotype A in 63% of patients with transient viremia and in 77% of those with persistent HBV viremia.
Mutations in the RT region of the HBV genome were detected in the four individuals assessed by sequencing. Although there were different combinations of mutations, complete resistance to lamivudine and partial resistance to entecavir was observed in all profiles. The most frequently encountered combination was rtL180 M + rtM204 V, which coincides with a previous Brazilian study.36
As for the amino acid patterns rtY126 and rtI296, which have been recently reported by Park et al.37 as associated with resistance to tenofovir for genotype C, there is so far no evidence that the same phenotype would occur in individuals infected with genotypes A or D. However, these profiles have been previously reported as characteristic of wild-type strains for HBV genotypes A1 and D2.13,38 We can thus hypothesize that in case these profiles are proven to reduce HBV genotype A and D sensitivity to tenofovir, likewise genotype C, a reduced genetic barrier to this antiretroviral should be considered for Brazilian patients.
Some limitations should be pointed out in our study: the single-center survey design and losses to follow-up or seroreversion. Although it does not present selection bias, as no significant difference was shown between included and eligible but nonincluded patients with respect to sex and age, the reduced sample may have compromised the statistical power of the study besides impairing the possibility to perform multivariate analysis. In addition, the cross-sectional design of our study precludes any conclusion on a causal link between the identified risk/protection factors and HBV viremia.
Nevertheless, our findings show the importance of adequate management of HIV/HBV-coinfected patients during long-term use of antiretroviral drugs. The need for strict control and long follow-up is emphasized, since even in the presence of undetected HIV RNA, HBV suppression may occur only after more than four years on proper antiviral chemotherapy. Moreover, in some coinfected individuals, it is possible to find sustained HBV viremia as consequence of poor adherence to therapy or emergence of drug-resistant mutants. Future analyses, preferably in multicenter studies with larger number of participants and longer follow-up, may contribute to better elucidate the virologic response of HIV/HBV-coinfected individuals on HBV therapy and its associated factors.
Conflicts of interestThe authors declare no conflicts of interest.