Lung transplantation has become the standard therapy for the treatment of eligible patients with terminal pulmonary disease. The last record published by the International Society for Heart and Lung Transplantation – Registry Report of 2018 showed that 64,803 lung transplantations had been performed up to June 2017. The mortality in the first 30 days is high and multifactorial: infections, acute rejection, graft failure 1and abdominal complication.2
In patients with cystic fibrosis (CF), abdominal complication are common in the immediate postoperative period.3 The most common early abdominal complication in these patients are distal intestinal obstruction syndrome (DIOS), gastroparesis, and Ogilve's syndrome that may need surgical intervention.2
We describe a case of a cystic fibrosis patient who underwent bilateral lung transplantation with intermittent obstructive abdominal pain and distension. Post mortem diagnosis was intestinal invasive mucormycosis without pulmonary involvement.4
A 25 year-old woman, diagnosed with CF at seven months of age and exocrine pancreatic insufficiency was included in the waiting list for lung transplant. She had airway colonization by Staphylococcus aureus methicillin-resistant (MRSA), Pseudomonas aeruginosa and Achromobacter sp. No fungal colonization was identified before the transplant; therefore no antifungal therapy was used.
She underwent bilateral sequential lung transplantation (LTx) without cardiopulmonary bypass. Left lung ischemic time was 315minutes and right lung 415minutes. The donor was a 15 year-old man with a traumatic brain injury as cause of death, no comorbidities, six days on mechanical ventilation, PaO2/FiO2 of 410 and no use of antibiotics.
Cultures of donor's bronchoalveolar lavage were all negative. Recipient's airway samples were also negative for fungal cultures and explanted lungs had no signs of fungal infection or colonization.
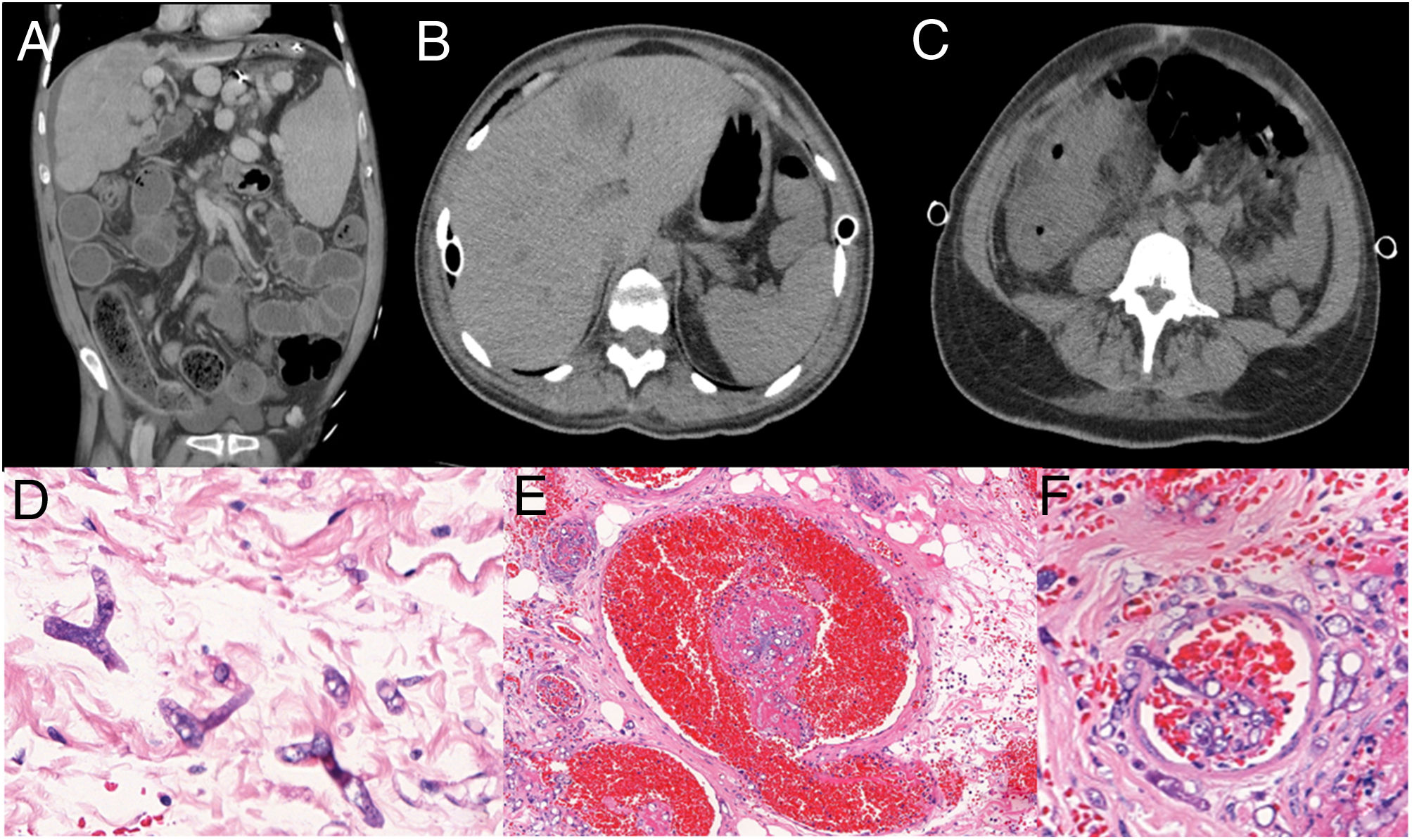
Post-LTx antimicrobial prophylaxis consisted of inhaled amphotericin and tobramycin, cotrimoxazol, and ganciclovir. Metilprednisolone and basiliximab were used as induction immunosuppression and maintenance was with cyclosporine, mycophenolate and prednisone. Patient also received teicoplanin and piperacillin-tazobactam according to pre-LTx bacterial colonization as well as fluconazole due to vulvovaginal candidiasis. She developed grade 3 primary lung graft dysfunction (PGD) and extubation was possible only on the 12th post-operative (PO) day. The first abdominal manifestation occurred on the 4th PO day with abdominal distension. Computed tomography (CT) of abdomen was performed and showed small bowel and colon distension only, no obstruction points were evidenced, cecum measuring 7cm and free fluid in the cavity. On the 9th PO day, she had a new abdominal distention episode. GI surgeons diagnosed metabolic ileus or DIOS (Figure 1A) for this intermittent abdominal pain and distension. Fleet enema, prokinetics, laxatives (polyethylene glycol) and parenteral diet improved abdominal distension and patient was discharged from the intensive care unit on 26th PO day.
A. Abdomen CT showing colonic wall thickening, mural striation, mesenteric soft-tissue infiltration, increased pericolonic fat mimetizing distal intestinal obstruction syndrome. B. Abdomen CT showing hepatic abscess; C. Increased densification of the colon. D. Large hyphae, rare septations and irregular ramifications at right or acute angle, thin, irregular walls. E. Thrombosis (mycotic thrombi), vascular necrosis and subsequent infarction, and ischemic/hemorrhagic necrosis of affected tissues. F. Angioinvasion, with thrombosis (mycotic thrombi). (D H&E 100x; E H&E 40x; F H&E 40x).
A new episode of abdominal pain and distension happened two days later. A new CT scan of the abdomen was performed (Figure 1B and 1C), showing hepatic abscess and increased densification of the colon and its surroundings. Patient rapidly developed renal dysfunction and metabolic acidosis. Anidulafungin substituted fluconazole and vancomycin plus meropenem were initiated on 31st PO day. Urgent exploratory laparotomy was performed, evidencing free fluid in the cavity, presence of right colon necrosis in the hepatic angle, liver and duodenal ischemia. Patient presented refractory shock with no response to the therapeutic interventions and died on immediate postoperative period.
The patient‘s family did not authorize necropsy but the pathology study of the laparotomy product showed: acute colitis with extensive necrosis of the intestinal wall and invasive fungal infection with morphological aspect of Mucorales order: large hyphae, rare septations and irregular ramifications at right or acute angle, thin, irregular walls (Figure 1D). Thrombosis (Figure 1E) and angioinvasion (Figure 1F), associated with discrete inflammatory tissue reaction. Lymphadenitis with numerous fungi in the subcapsular sinuses. Acute extensive ulcerated ileitis and fungal infection. Liver necrosis with diffuse fungal infiltration.
This case is emblematic because the post-LTx period has many common but misleading events: primary graft dysfunction, antibiotics, immunosuppressive therapy, and abdominal manifestations misdiagnosed due to the underlying lung disease. The patient presented an intermittent abdominal pain and distension that the LTx team thought about innumerous hypotheses, including DIOS. Ischemic bowel disease is one of the differential diagnosis and may be due to invasive fungal infection such as mucormycosis
The gastrointestinal manifestations in CF patients include pancreatic insufficiency, DIOS and biliary tract complications, which can lead to cirrhosis and hepatic failure.3,5 Gastrointestinal mucormycosis in immunocompromised hosts 6also have similar clinical manifestations, such as abdominal pain (68%), gastrointestinal bleeding (48%), fever (19%), change in bowel habits (10%). Furthermore, only 25% of all GI mucormycosis have bowel involvement alone among solid organ transplant recipients. There are only few case reports and most of then confirmed only at autopsy, just like our case.4 Gastrointestinal mucormycosis is a rare condition after solid organ transplantation. Herein we report a case of invasive fungal infection in small bowel and liver without coinfection with other fungi and no evidence of explanted lung infection.7
The diagnosis of gastrointestinal mucormycosis is delayed because of its nonspecific presentation and requires a high degree of suspicion, leading to early biopsy.
In most cases, with negative fungal culture, histopathologic sample is enough to establish the fungal invasive diagnosis.8,9 However, histopathologic examination is suggestive, but not confirmatory, as the morphology of fungi in tissue may be difficult to interpret.9 Presence of a thick, non-septate hypha branching at right angle on histopathological examination is specific for mucormycosis. Molecular techniques can be used to confirm the diagnosis, although there are no standardized molecular tests currently available. Galactomannan and β-D-glucan test do not detect antigen components of Mucorales cell wall.10
Mucormycosis has emerged as an important fungal infection with high associated mortality rates. Although any organ system may be affected, the most common reported sites of invasive mucormycosis are paranasal sinuses (39%), lungs (24%), and skin (19%). Mucormycosis restricted to the gastrointestinal tract is uncommon and accounts for only 7% of cases.11
Mucormycosis confined to gastrointestinal tissue accounts for only 4%–7% of all documented cases.12 Gastrointestinal mucormycosis has a high mortality and 85% of deaths are related to bowel perforation and upper gastrointestinal hemorrhage.13 However, when the infection is disseminated, mortality is 100%, such as in our case which involved the liver, lymph nodes and small bowel.
Mucormycosis in the gastrointestinal tract has not been described in the literature in a post-lung transplant patient without pulmonary involvement. However, there are two 14,15cases described after cardiopulmonary transplantation. The similarities with our case are gastrointestinal tract involvement and non-specific signs and symptoms, and surgery intervention was also required but with unfavorable outcome.
Mucormycosis is a rare invasive fungal infection that mainly affects immunocompromised patients. LTx recipients, specially with CF, have many early post-operative abdominal manifestation and physicians should be aware of the possibility of gastrointestinal mucormycosis because of the non-specific clinical manifestations and high mortality rate attributed to this infection.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors declare no conflicts of interest.