There has been a significant decrease in the number of approved antibiotics in the last two decades, and in parallel, a steady increase of multidrug resistant bacteria (MDR) has been occurring. Thus, MDR have become a global issue of public health, and with this threat, the challenge to develop new antibiotics has emerged in all areas: governmental, scientific, and the private pharmacological industry.1 In this sense, drug repositioning has arisen as an alternative approach for the faster identification of drugs that are effective against infectious diseases.2
The expressions “Drug repositioning” and “drug repurposing” was first described by Ashburn and Thor (2004)3 in their paper “Drug repositioning: identifying and developing new uses for existing drugs”. According to the authors, this is the process to find new uses for clinically approved drugs, and this is also known as redirecting and reprofiling.
Several studies have signalled that drug repositioning has advantages compared to the traditional way of seeking for active substances,2,4–7 since pharmacological, toxicological and bioavailability data, among others, are already available. Thus, less time is spent in their development, leading to a significant reduction in costs, and it proves to be a preferred and advantageous alternative strategy to discover drugs more quickly.4 Other encouraging data are the success rates for repositioned drugs, which are higher when compared to new drugs, reaching 30% in the last few years. Also, together with the positive aspects of repositioning is its recent approval by the Food and Drug Administration (FDA).8
Comparing repurposing and use off-label, there is a similarity between these practices: a new indication of the drug, other than the usual one. However, the use outside the label goes beyond this, since it may include different age groups, dosage or route of administration. Although this is considered a legal and common application, it is often performed in the absence of adequate scientific data, and may expose patients to unrestricted and ineffective experimentation of drugs with unknown health risks.9
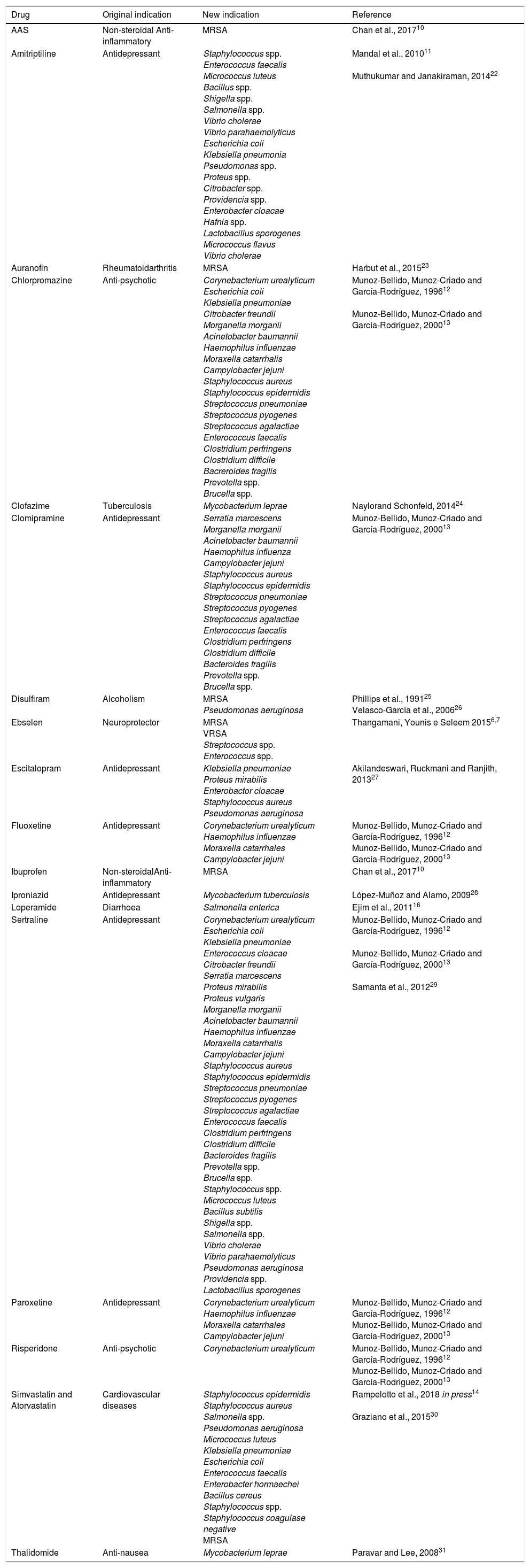
In Table 1, we present a summary of the repositioning of drugs for antibacterial treatment: examples of studies that investigate the antimicrobial activities of several pharmacological classes, including psychotropics, local anaesthetics, tranquilizers, cardiovascular drugs, antihistamines, anti-inflammatories, being these called “non-antibiotic drugs”.10–14
Studies of repositioning non-antibiotic drugs with antibiotic effect.
| Drug | Original indication | New indication | Reference | |
|---|---|---|---|---|
| AAS | Non-steroidal Anti-inflammatory | MRSA | Chan et al., 201710 | |
| Amitriptiline | Antidepressant | Staphylococcus spp. Enterococcus faecalis Micrococcus luteus Bacillus spp. Shigella spp. Salmonella spp. Vibrio cholerae Vibrio parahaemolyticus Escherichia coli Klebsiella pneumonia Pseudomonas spp. Proteus spp. Citrobacter spp. Providencia spp. Enterobacter cloacae Hafnia spp. Lactobacillus sporogenes Micrococcus flavus Vibrio cholerae | Mandal et al., 201011 Muthukumar and Janakiraman, 201422 | |
| Auranofin | Rheumatoidarthritis | MRSA | Harbut et al., 201523 | |
| Chlorpromazine | Anti-psychotic | Corynebacterium urealyticum Escherichia coli Klebsiella pneumoniae Citrobacter freundii Morganella morganii Acinetobacter baumannii Haemophilus influenzae Moraxella catarrhalis Campylobacter jejuni Staphylococcus aureus Staphylococcus epidermidis Streptococcus pneumoniae Streptococcus pyogenes Streptococcus agalactiae Enterococcus faecalis Clostridium perfringens Clostridium difficile Bacreroides fragilis Prevotella spp. Brucella spp. | Munoz-Bellido, Munoz-Criado and García-Rodríguez, 199612 Munoz-Bellido, Munoz-Criado and García-Rodríguez, 200013 | |
| Clofazime | Tuberculosis | Mycobacterium leprae | Naylorand Schonfeld, 201424 | |
| Clomipramine | Antidepressant | Serratia marcescens Morganella morganii Acinetobacter baumannii Haemophilus influenza Campylobacter jejuni Staphylococcus aureus Staphylococcus epidermidis Streptococcus pneumoniae Streptococcus pyogenes Streptococcus agalactiae Enterococcus faecalis Clostridium perfringens Clostridium difficile Bacteroides fragilis Prevotella spp. Brucella spp. | Munoz-Bellido, Munoz-Criado and García-Rodríguez, 200013 | |
| Disulfiram | Alcoholism | MRSA Pseudomonas aeruginosa | Phillips et al., 199125 Velasco-García et al., 200626 | |
| Ebselen | Neuroprotector | MRSA VRSA Streptococcus spp. Enterococcus spp. | Thangamani, Younis e Seleem 20156,7 | |
| Escitalopram | Antidepressant | Klebsiella pneumoniae Proteus mirabilis Enterobactor cloacae Staphylococcus aureus Pseudomonas aeruginosa | Akilandeswari, Ruckmani and Ranjith, 201327 | |
| Fluoxetine | Antidepressant | Corynebacterium urealyticum Haemophilus influenzae Moraxella catarrhales Campylobacter jejuni | Munoz-Bellido, Munoz-Criado and García-Rodríguez, 199612 Munoz-Bellido, Munoz-Criado and García-Rodríguez, 200013 | |
| Ibuprofen | Non-steroidalAnti-inflammatory | MRSA | Chan et al., 201710 | |
| Iproniazid | Antidepressant | Mycobacterium tuberculosis | López-Muñoz and Alamo, 200928 | |
| Loperamide | Diarrhoea | Salmonella enterica | Ejim et al., 201116 | |
| Sertraline | Antidepressant | Corynebacterium urealyticum Escherichia coli Klebsiella pneumoniae Enterococcus cloacae Citrobacter freundii Serratia marcescens Proteus mirabilis Proteus vulgaris Morganella morganii Acinetobacter baumannii Haemophilus influenzae Moraxella catarrhalis Campylobacter jejuni Staphylococcus aureus Staphylococcus epidermidis Streptococcus pneumoniae Streptococcus pyogenes Streptococcus agalactiae Enterococcus faecalis Clostridium perfringens Clostridium difficile Bacteroides fragilis Prevotella spp. Brucella spp. Staphylococcus spp. Micrococcus luteus Bacillus subtilis Shigella spp. Salmonella spp. Vibrio cholerae Vibrio parahaemolyticus Pseudomonas aeruginosa Providencia spp. Lactobacillus sporogenes | Munoz-Bellido, Munoz-Criado and García-Rodríguez, 199612 Munoz-Bellido, Munoz-Criado and García-Rodríguez, 200013 Samanta et al., 201229 | |
| Paroxetine | Antidepressant | Corynebacterium urealyticum Haemophilus influenzae Moraxella catarrhales Campylobacter jejuni | Munoz-Bellido, Munoz-Criado and García-Rodríguez, 199612 Munoz-Bellido, Munoz-Criado and García-Rodríguez, 200013 | |
| Risperidone | Anti-psychotic | Corynebacterium urealyticum | Munoz-Bellido, Munoz-Criado and García-Rodríguez, 199612 Munoz-Bellido, Munoz-Criado and García-Rodríguez, 200013 | |
| Simvastatin and Atorvastatin | Cardiovascular diseases | Staphylococcus epidermidis Staphylococcus aureus Salmonella spp. Pseudomonas aeruginosa Micrococcus luteus Klebsiella pneumoniae Escherichia coli Enterococcus faecalis Enterobacter hormaechei Bacillus cereus Staphylococcus spp. Staphylococcus coagulase negative MRSA | Rampelotto et al., 2018 in press14 Graziano et al., 201530 | |
| Thalidomide | Anti-nausea | Mycobacterium leprae | Paravar and Lee, 200831 | |
MRSA, methicillin-resistant Staphylococcus aureus; VRSA, vancomycin-resistant Staphylococcus aureus; AAS, acetylsalicylic acid.
The treatment of chronic bacterial infections in immunocompromised patients with synergistic drug combinations is well established, and this procedure has been used for several years.15 These synergistic combinations are used because of three main advantages: expansion of the antibiotic spectrum16,17; overcoming resistance18; and decrease of resistance to antibiotics through their careful use.19–21
Since repositioned non-antibiotic drugs have shown antibiotic effects among themselves as well as when used together with antimicrobials, these combinations presently consist of a useful option to overcome the problem of weak activity of individual drugs.2,10,16
Based on the several studies presented, it can be inferred that the repositioning of non-antibiotic drugs with known toxicity profiles represents a promising alternative for the treatment of bacterial infections. Nevertheless, it is a consensus in the global scientific community that it is only the starting point, and additional studies regarding mechanisms of action and in vivo studies, among others, are vital for the safe use of these drugs.
Conflicts of interestThe authors declare no conflicts of interest.