This study aims to explore the epidemiology, clinical profile and strain characteristics of cryptococcosis from 2013 to 2017 in a major teaching hospital in China.
MethodsTrends in antifungal drug susceptibility of 217 consecutive non-repetitive cryptococcal isolates collected from patients of an university hospital in China were analyzed between 2013 and 2017. Of those, 98 isolates were conserved for identification by internal transcribed spacer (ITS) sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) system. Multilocus sequence typing (MLST) was used to designate molecular types. Clinical characteristics of the 98 patients with cryptococcosis during the period of 2013–2017 were retrospectively evaluated.
ResultsThere was a trend for gradual increase in the MIC range of fluconazole was from 2013 to 2017. The conserved 98 clinical cryptococcal isolates included 97 C. neoformans and one C. gattii, and 90 (91.8%) isolates belonged to ST5 genotype VNI. Out of the 98 patients with cryptococcosis, 28 (28.6%) were HIV-infected and 32 (32.7%) had no underlying diseases. HIV-infected patients had higher mortality than HIV-uninfected patients (28.6% vs 14.3%, p=0.147).
ConclusionsMost of the patients with cryptococcosis were not HIV-infected in this study, while patients with HIV had a higher mortality. Reduced susceptibility to fluconazole was observed among C. neoformans isolates, most of them belonged to ST5 genotype VNI having an impact on the effective dose of fluconazole.
Cryptococcosis is a significant invasive fungal infection with noteworthy morbidity and mortality, primarily caused by Cryptococcus neoformans (C. neoformans)and Cryptococcus gattii.1,2 Although cryptococcosis may occur in healthy hosts,3 the majority of infections occur in patients with significant underlying predisposing factors, such as organ transplantation, hematologic malignancies, and advanced human immunodeficiency virus (HIV) disease.2,4–8 In Europe and the in United States about 80% of the cryptococcosis cases were associated with HIV/AIDS.2,9 Nonetheless, in China, the majority of patients with cryptococcosis are not infected with HIV.10,11 This infection has been increasing steadily over the past years in China.
In recent years, there is a growing concern among clinicians regarding the potential for the emergence of antifungal resistance among C. neoformans because of high rates of fungal persistence and frequent disease relapse.12–14 Several reports on the emergence of resistance to amphotericin B, fluconazole, flucytosine, or itraconazole in C. neoformans during treatment has been published.15,16 Consequently, widespread increase in fluconazole resistance among C. neoformans became a public health concern calling for more-widespread surveillance. However, studies on the trends in antifungal drug susceptibility of Cryptococcus are limited in China. In 2016, Fan et al. reported a proportional increase of cryptococcal isolates with non-wild-type MICs to fluconazole (non-WT, MIC>8μg/ml) in China from 2009 to 2014,15 but clinical characteristics of these cryptococcal isolates were not collected. Now, the trend in antifungal drug susceptibility of clinical Cryptococcus strains in China is still uncertain, and further research on the correlation of the antifungal drug susceptibility trend and clinical outcomes is necessary.
In this study, we analyzed the trends in antifungal drug susceptibility of 217 consecutive cryptococcal isolates from a medical center in China between 2013 and 2017, of which 98 isolates were conserved for molecular evaluation. The present study also assessed the clinical manifestations of the 98 patients during the study period.
Material and methodsCryptococcal isolatesThis was a cross-sectional retrospective study in a 2500-bed teaching hospital in east China. A total of 217 non-repetitive Cryptococcus clinical strains were consecutively isolated from Cryptococcus culture-proven patients from 2013 to 2017 in this hospital. Antifungal drug susceptibility was detected for all 217 clinical strains.
Out of these 217 strains, 98 isolates were randomly selected and stored in 25% glycerol at –80°C until use and were maintained on yeast peptone dextrose (1% yeast extract, 2% peptone, 2% glucose) agar at 25°C for species identification and MLST during this study.
Antifungal susceptibility studiesThe antifungal drug susceptibility of the total 217 Cryptococcus strains was detected by ATB FUNGUS 3 kit (BioMérieux France). Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were used as quality control organisms. The interpretive criteria for susceptibility to fluconazole and flucytosine were according to the manufacturer’s instructions of ATB FUNGUS 3 kit. For fluconazole: susceptible, 4μg/ml; susceptible dose dependent, 8μg/ml; resistant, 16μg/ml; and for flucytosine, susceptible, 4μg/ml; intermediate, 8–16μg/ml; resistant, 32μg/ml. As there are currently no standard clinical breakpoints for Cryptococcus spp., we used the epidemiological cut-off values as recommended by previous studies: voriconazole, 0.125μg/ml; itraconazole, 0.25μg/ml; and amphotericin B, 1μg/ml.15,17,18
DNA extractionAmong the 98 conserved clinical cryptococcal strains genomic DNA was extracted usin a commercial kit (Sangon Biotech Co., Ltd, Shanghai, China).
Identification of Cryptococcus species and varietyAmplification of the rDNA internal transcribed spacer (ITS) region was performed as previously described.15,19 The PCR products were sequenced in both directions using the DNA analyzer ABI 3730XL system (Applied Biosystems, Foster City, CA). The obtained ITS sequences of Cryptococcus isolates were compared against those contained in the Centraalbureau voor Schimmelcultures (CBS) Fungal Biodiversity Centre database by using BIOLOMICSNET software (http://www.cbs.knaw.nl/collections/BioloMICSSequences.aspx).
Bruker Biotyper MALDI-TOF MS (Bruker, Daltonik, Bremen, Germany) was also used to identify the conserved 98 Cryptococcus clinical strains. According to the manufacture’s recommendations and as Buchan et al. described, 16 identification by plate-grown isolates (on-plate protein extraction method) was applied. The procedure was performed as previously reported.15
Multilocus sequence typing (MLST) and mating type analysisFor MLST analysis, DNA fragments of seven unlinked genes that included IGS1, GPD1, CAP59, URA5, PLB1, LAC1, and SOD1 were amplified by PCR from the 98 preserved Cryptococcus clinical strains. Alleles and sequence types (STs) were assigned by using the fungal MLST database (http://mlst.mycologylab.org/). Molecular types (i.e., VNI to VNIV for C. neoformans and VGI to VGIV for C. gattii) were assigned according to isolates' STs and were queried against the online MLST database (http://mlst.mycologylab.org).
Patient selection and data collectionClinical characteristics of the 98 cryptococcosis patients with positive Cryptococcus culture were retrospectively evaluated. Demographic and clinical data including predisposing factors, infection types and outcome, were abstracted from the electronic record.
Statistical analysisThe Statistical Package for the Social Sciences (SPSS, Version 22.0, Chicago, IL, USA) was used for the analysis. Two groups (HIV and non-HIV patients) were specified and investigated. Descriptive data are reported as either mean±SD, median (interquartile range) or number and percentage. Difference between continuous variables was compared by Student's t-test and non-parametrical tests. Categorical variables were compared by chi-square analysis. A p<0.05 was considered statistically significant.
Ethics and consent statementsThis was a retrospective study. All isolates present in this study had been stored in the Department of Microbiology, the First Affiliated Hospital, Zhejiang University School of Medicine, China. The Institutional Review Board of the First Affiliated Hospital, College of Medicine, Zhejiang University waived the need for informed consent and granted permission to use the strains to perform all experiments in this study.
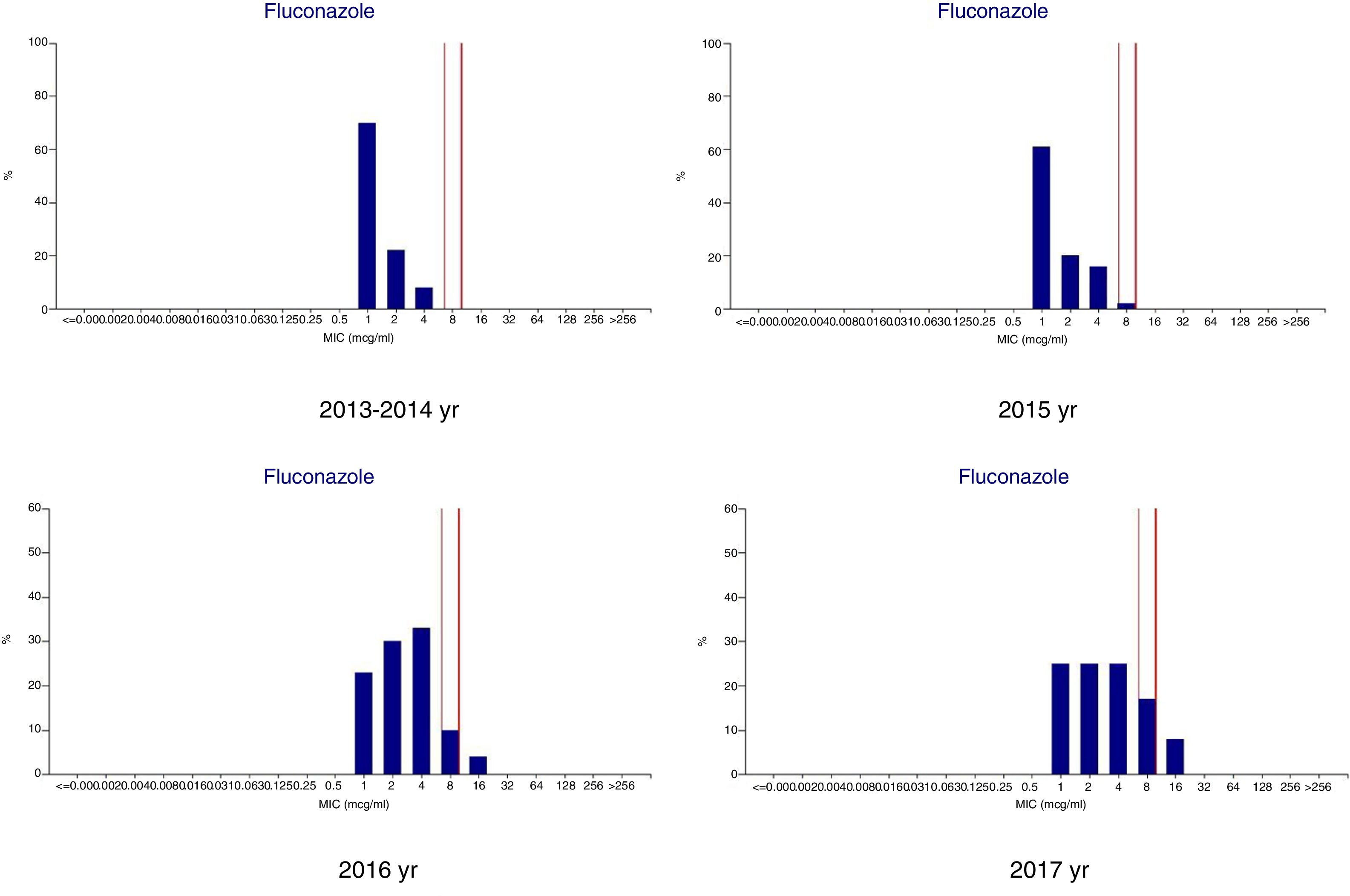
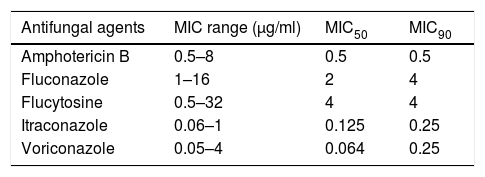
ResultsMicrobiologic characterization of the cryptococcal isolatesTrends in antifungal drug susceptibility of 217 cryptococcal isolates from 2013 to 2017Table 1 summarizes the in vitro susceptibilities to the five antifungal agents in use (amphotericin B, fluconazole, flucytosine, itraconazole, and voriconazole) of the 217 Crypyococcus isolated at an university hospital between 2013 and 2017. All of the 217 Crypyococcus isolates were highly susceptible to amphotericin B (99.1% susceptible) over the entire 5-year period. Also, it was shown that susceptibility to fluconazole decreased from 1.0% in 2013 to 75.0% in 2017. The MIC range of fluconazole showed gradual increasing trend shifting from 1 to 4μg/ml in 2013–2014 to 1–8μg/ml in 2015, and to 1–16μg/ml in 2016–2017 (Fig. 1).
In vitro susceptibility to five antifungal agents of 217 cryptococcal isolates from an university hospital during a 5-year period (2013–2017).
| Antifungal agents | MIC range (μg/ml) | MIC50 | MIC90 |
|---|---|---|---|
| Amphotericin B | 0.5–8 | 0.5 | 0.5 |
| Fluconazole | 1–16 | 2 | 4 |
| Flucytosine | 0.5–32 | 4 | 4 |
| Itraconazole | 0.06–1 | 0.125 | 0.25 |
| Voriconazole | 0.05–4 | 0.064 | 0.25 |
According to MALDI-TOF MS results, the conserved 98 clinical cryptococcal strains included 97C. neoformansstrains (96 var. grubii strains and one var. neoformans strain) and one C. gattii strain, which are consistent with the rDNA ITS region amplification results. rDNA ITS region amplification are in agreement with those of MALDI-TOF MS.
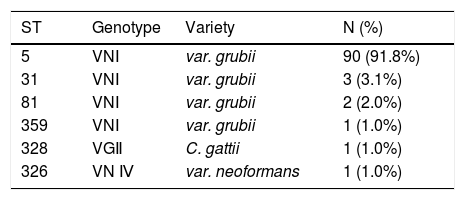
MLST and molecular type results of the 98 clinical cryptococcal isolates are shown in Table 2. MLST analysis revealed that all 98 cryptococcal isolates belonged to six sequence types, including ST5 (90 isolates, 91.8%), ST31 (3 isolates, 3.1%), ST81 (2 isolates, 2.0%), ST326 (1 isolates, 1.0%), ST328 (1 isolates, 1.0%), and ST359 (1 isolates, 1.0%). Molecular type VNI (var. grubii) was recovered in 96 clinical cryptococcal isolates belonging to ST5, ST31, ST81, and ST359. The type VGII (C. gattii) was found in one clinical cryptococcal isolate belonging to ST328. While the type VNⅣ (var. neoformans) was found in one clinical cryptococcal isolate belonging to ST326.
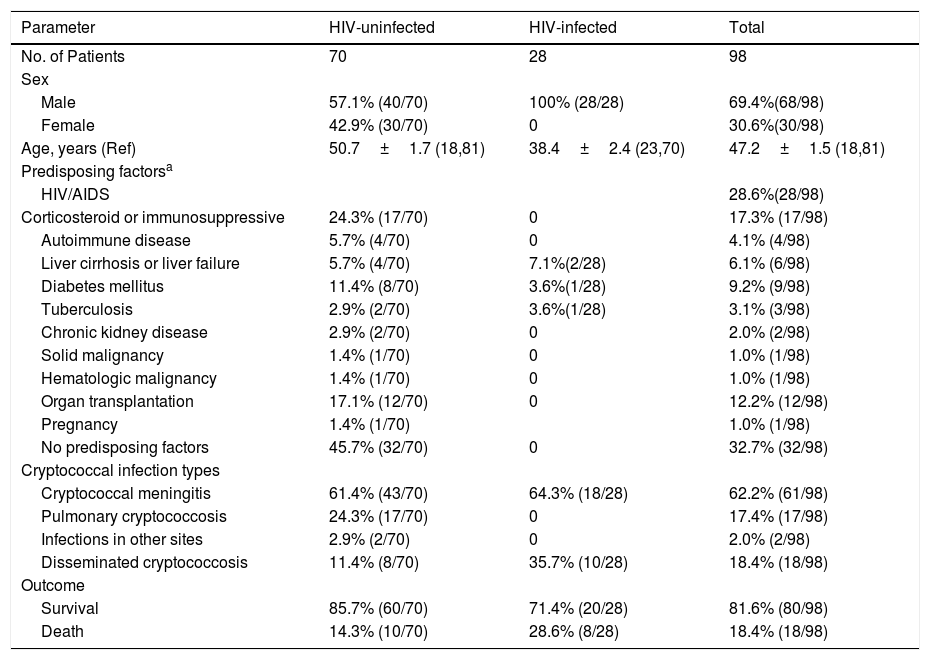
Patients’ characteristicsDemographic data and clinical characteristics of the 98 Cryptococcus culture-proven patients of the study are shown in Table 3. Among the 98 patients with cryptococcosis, 28 (28.6%) were male HIV-infected patients and 70 (71.4%) non-HIV-infected patients. There was no apparent sex difference in non-HIV-infected patients with cryptococcosis. HIV-infected patients were younger than non-infected patients (38.4±2.4 vs 50.7±1.7 years). Seventeen (13.7%) patients were on corticosteroids or immunosuppressives, 12 (12.2%) patients hag undergone organ transplantation, diabetes mellitus was found in nine (9.2%) patients, but 32 (32.7%) had no underlying diseases.
Demographic features and underlying diseases of HIV-infected and uninfected cryptococcosis patients from hospital-based studies.
| Parameter | HIV-uninfected | HIV-infected | Total |
|---|---|---|---|
| No. of Patients | 70 | 28 | 98 |
| Sex | |||
| Male | 57.1% (40/70) | 100% (28/28) | 69.4%(68/98) |
| Female | 42.9% (30/70) | 0 | 30.6%(30/98) |
| Age, years (Ref) | 50.7±1.7 (18,81) | 38.4±2.4 (23,70) | 47.2±1.5 (18,81) |
| Predisposing factorsa | |||
| HIV/AIDS | 28.6%(28/98) | ||
| Corticosteroid or immunosuppressive | 24.3% (17/70) | 0 | 17.3% (17/98) |
| Autoimmune disease | 5.7% (4/70) | 0 | 4.1% (4/98) |
| Liver cirrhosis or liver failure | 5.7% (4/70) | 7.1%(2/28) | 6.1% (6/98) |
| Diabetes mellitus | 11.4% (8/70) | 3.6%(1/28) | 9.2% (9/98) |
| Tuberculosis | 2.9% (2/70) | 3.6%(1/28) | 3.1% (3/98) |
| Chronic kidney disease | 2.9% (2/70) | 0 | 2.0% (2/98) |
| Solid malignancy | 1.4% (1/70) | 0 | 1.0% (1/98) |
| Hematologic malignancy | 1.4% (1/70) | 0 | 1.0% (1/98) |
| Organ transplantation | 17.1% (12/70) | 0 | 12.2% (12/98) |
| Pregnancy | 1.4% (1/70) | 1.0% (1/98) | |
| No predisposing factors | 45.7% (32/70) | 0 | 32.7% (32/98) |
| Cryptococcal infection types | |||
| Cryptococcal meningitis | 61.4% (43/70) | 64.3% (18/28) | 62.2% (61/98) |
| Pulmonary cryptococcosis | 24.3% (17/70) | 0 | 17.4% (17/98) |
| Infections in other sites | 2.9% (2/70) | 0 | 2.0% (2/98) |
| Disseminated cryptococcosis | 11.4% (8/70) | 35.7% (10/28) | 18.4% (18/98) |
| Outcome | |||
| Survival | 85.7% (60/70) | 71.4% (20/28) | 81.6% (80/98) |
| Death | 14.3% (10/70) | 28.6% (8/28) | 18.4% (18/98) |
Among the 70 non-HIV-infected patients with cryptococcosis, 43 (61.4%) patients were diagnosed as cryptococcal meningitis, 17 (17/70, 24.3%) with pulmonary cryptococcosis, and eight (11.4%) patients with disseminated cryptococcosis. However, the incidence rate of disseminated cryptococcosis among the 28 HIV-infected patients was 35.7% (10/28), significantly higher than that in the 70 non-HIV-infected group (p<0.05). Except for the 10 HIV-infected patients infected with disseminated cryptococcosis, the remaining 18 HIV-infected patients were all diagnosed as cryptococcal meningitis. The overall mortality rate was 18.4%, but higher among HIV-infected patients (28.6%) than in non-HIV-infected patients (p=0.147), Table 3.
DiscussionIn the past years, the incidence of cryptococcosis in China is on the rise, and the fungal persistence and frequent disease relapse of cryptococcosis is the main clinical problem. Several reports of the emergence of resistance to the antifungal agents in HIV-infected patients have been published.12,16,20 However, in China, cryptococcosis mostly occurs in patients without HIV infection, and there are limited data on the molecular epidemiology of cryptococcosis in China. In our study, the susceptibility to the five antifungal agents in use (amphotericin B, fluconazole, flucytosine, itraconazole, and voriconazole) of 217 cryptococcal isolates from patients admitted to an university hospital between 2013 and 2017 were evaluated. The trend in the MIC range of fluconazole showed gradual increase from 2013 to 2017, while 16 C. neoformans strains were dose-dependent susceptible to fluconazole (MIC 8μg/ml) and five C. neoformans strains with non-wild-type MICs to fluconazole (MIC 16μg/ml) were detected in 2016–2017. This phenomenon was worth to be conveyed to clinicians, especially in cryptococcosis cases with fungal persistence and relapse. The Infectious Diseases Society of America (IDSA) recommends amphotericin B and 5-flucytosine as the preferred agents for the induction therapy, whereas the azoles (especially fluconazole) are used in the consolidation and maintenance phases of therapy, or as primary prophylaxis.21 However, in resource limited settings, azoles are often used as initial therapy.22 In the past years in our hospital, a relatively low fluconazole dose (400mg per day and even 200mg per day) was commonly administrated for therapy or prophylaxis of fungal infection, which might lead to the gradual upward MIC drifts of fluconazole. In several reports, high-dose of fluconazole (800mg per day or even 1200mg per day) was confirmed more efficacious to treat cryptococcosis.22,23 Higher doses of fluconazole and longer durations of treatment are suggested.21,23 The gradual upward MIC drifts of fluconazole might also explain the reason that some patients with cryptococcosis were cured or improved with higher dose of fluconazole. Amphotericin B maintained 1.0% susceptibility in the period of 2013–2016, but one isolate was shown to be resistant to amphotericin B (MIC 8μg/ml) in 2017.
As the results of species and varieties identification by MALDI-TOF MS and ITS sequencing, the conserved 98 clinical cryptococcal strains included 97 C. neoformansstrains (96 var. grubii strains and one var. neoformans strain) and one C. gattii strain. Analysis of MLST showed that 91.8% of the isolates belonged to ST5 genotype VNI, which was the main ST of clinical C. neoformans in China, consistent with the related reports.15C gattii, an emerging agent of cryptococcosis, was initially reported only in tropical and subtropical zones and rarely reported in China.24 The patient infected with C. gattii had been worked in Cambodia and suffered before going back to China. So, this case of C. gattii cryptococcosis might have been imported.
In previous reports, about 80% of the cryptococcosis cases were associated with HIV/AIDS in Europe and the United States.9,25 In contrast, in our study most of patients (71.4%) with cryptococcosis were not HIV-infected; moreover, 32.7% of the cryptococcosis patients had no underlying diseases, as shown in other reports from China.6,26 All of the 28 HIV-infected cryptococcosis patients with were male, and younger than the patients without HIV. There was no apparent sex difference in the cryptococcosis patients without HIV. The incidence rate of disseminated cryptococcosis, as well as the mortality rate, among HIV-infected patients were significantly higher than that in patients without HIV infection.
In contrast to the findings of Lee et al., no relationship between MICs of antifungal agents and outcome of patients with cryptococcosis could be demonstrated in this study. The discrepancy might be due to different choices of treatment regimens.14 In this study, four of the five patients infected with C. neoformans with higher MICs for fluconazole were still cured or improved following the regimen recommended by IDSA with amphotericin B and 5-flucytosine as the preferred agents for the induction therapy and fluconazole or voriconazole used in the consolidation and maintenance phases. Moreover, the patient infected with C. neoformans resistant to amphotericin B (MIC 8μg/ml) responded to voriconazole. However, the shifting trend in MIC ranges of antifungal agents, especially fluconazole, warrants attention as it was also reported in other countries and might be related to the dose selection of fluconazole for effective therapy.27 Hence, the dynamic real time surveillance of susceptibility for antifungal agents is necessary in clinic practice.
This study has some limitations. It was a single center retrospective study conducted in a major teaching hospital in China. Hence, the results in this study are exploratory. In future, further investigations with larger samples including more hospitals in China will be necessary to generalize the results for the Chinese population.
DisclosureThe authors report no conflicts of interest in this work.