A population survey was conducted to explore the prevalence and factors associated with Leishmania infection in the Fercal region of the Federal District. The Fercal region is a group of neighborhoods in Brasília in which the first cases of visceral leishmaniasis were described. Leishmania infection was established by a positive leishmanin test. Although other tests were performed in the study (an immunochromatographic assay (Kalazar detect®) and a molecular assay), only the leishmanin skin test provided sufficient results for the measurement of the disease prevalence. Data on the epidemiological, clinical and environmental characteristics of individuals were collected along with the diagnostic tests. After sampling and enrollment, seven hundred people from 2 to 14 years of age were included in the study. The prevalence of Leishmania infection was 33.28% (95% CI 29.87–36.84). The factors associated with Leishmania infection according to the multivariate analysis were age of more than seven years and the presence of opossums near the home. Age is a known factor associated with Leishmania infection; however, the presence of wild animals, as described, is an understudied factor. The presence of opossums, which are known reservoirs of Leishmania, in peri-urban areas could be the link between the rural and urban occurrence of visceral leishmaniasis in the outskirts of largest Brazilian cities, as suggested by previous studies.
Visceral leishmaniasis (VL) is an expanding zoonosis in Brazil and Latin America. The infection has progressed from rural areas to the suburbs of large cities; there is a possibility of humans becoming infected in the urban areas of some of the largest cities in Brazil. The urbanization of VL is well described.1 The first Brazilian cities to experience this phenomenon have experienced 40 years of VL endemicity,2 and the first autochthonous cases of VL in Brasília, occurring in the Federal District, were described in 2005 by our group.3
In Brazil, several studies have failed to detect the factors associated with positive testing for Leishmania.4–8 A recent meta-analysis reviewed Latin-American publications, most of which were Brazilian, looking for factors associated with VL and described an association of older age, presence of dogs in the house, higher canine seropositivity in nearby areas, lower socioeconomic status, and highly vegetated areas with Leishmania (L.) infantum infection. The association of Leishmania infection and gender varied according to the test performed. The factors associated with seropositivity were not identical to those associated with delayed skin test reactivity.9Leishmania infection was found to be significantly associated with variables such as living in the same house of a VL patient, having relatives with LV, or being over 23 months of age.10–13 Most of these studies were conducted in areas with a lengthy endemicity of VL. As noted by Belo et al., many studies assessing the risk factors for VL are questioned on the basis of a number of factors, such as the reliance on small or poorly representative samples, the strategies to avoid refusals or not describing the magnitude of losses, the use of combined diagnostic tests for diagnosing infections, and the control of confounding variables and issues related to the statistical analysis.
This study aims to describe the magnitude and factors associated with Leishmania infection in the area of Fercal, a conglomeration of neighborhoods in which we previously described the first human cases of VL in Brasília and from which most of the cases of the disease were reported between 2005 and 2010.
Patients, material and methodsThis research was an observational, cross-sectional analytical study aiming to estimate the prevalence of Leishmania infection in the studied area.
Study area and populationThe area identified as Fercal, is located in the administrative region of Sobradinho II in the outskirts of Brasília, Brazil. Between 2005 and 2010, this region contributed more than half of the cases of autochthonous VL in the Federal District. According to census, the population of Brasília was 2455.903 inhabitants in 2010, and Fercal estimated population was 32,000 people. Fercal comprises several communities separated by mountainous areas of residual forest and farms located in a area rich in vegetation and water sources. The occupation of this region has gradually occurred for about 60 years, originating near a limestone factory. The study was conducted between 2007 and 2008 and covered the communities of Alto Bela Vista, Bananal, Boa Vista, Catingueiro, Córrego do Ouro, Curvas, Engenho Velho, Fercal I and II, Queima Lençol, Ribeirão-Pedreira, and Rua do Mato, all of which are in the Fercal region.
Sample sizeAssuming that there were approximately 5000 residents within the chosen age range, an estimated 15% prevalence rate of infection, a precision of 5%, a sample size of 134 people would be necessary to be included in the study. Because this study would be the beginning of a follow-up study, the sample size was inflated by a factor of 5 and rounded to 700 participants. The number of subjects from each community was calculated taking into account the number of households in that community, so that 73 individuals from Alto Bela Vista, 132 from Bananal, 80 from Boa Vista, 20 from Córrego do Ouro, 65 from Curvas, 117 from Engenho Velho, 66 from Fercal I, 27 from Fercal II, 57 from Queima Lençol, 16 from Ribeirão-Pedreira and 47 from Rua do Mato were included.
RandomizationIn each participating community the houses to be contacted were randomly selected. Only one participant from each house was included. Houses without individuals in the target age range or in case participation in the study was not accepted another house was randomized in the same community.
Inclusion criteriaThe study included Fercal residents aged two to 14 years, who fulfilled the following inclusion criteria and agreed to participate in the study after approval from their parents or guardians. The following inclusion criteria were used: residing continuously for more than one year in the study area, no history of symptomatic leishmaniasis, and no contraindications to blood extraction or to administration of a leishmanin skin test (LST).
Laboratory testsThe leishmanin test: Montenegro's antigen contains a lysate of promastigotes of L. (L.) amazonensis, strain MHOM/BR/73/M2269, batch 01/07 manufactured by the Centro de Produção e Pesquisa de Imunobiológicos (CPPI) from the Instituto de Saúde do Paraná. For the test, 0.1mL of the suspension was intradermally inoculated in the forearm. The reading was performed 48–72h after the inoculation by measuring the diameter (in millimeters) of dermal induration with the ballpoint-pen technique. An induration equal or superior to 5mm was considered positive. The immunochromatographic assay Kalazar detect® (InBios International, Seattle, WA) was performed on serum samples of the included participants. The assay is based on the color shift due to the presence of antibodies against the recombinant K39 antigen. The procedures were performed according to the manufacturer's indications. The serum and buffer solution were tested at room temperature using an ELISA plate as a base. Ribbons and 20μL of serum were placed in the wells of the plate. Three drops of buffer solution were added to each well, and the reading was conducted after 10min of reaction. Positivity was considered when two lines were evident (control and positive). Polymerase chain reactions were performed on samples of 300μL of whole blood. Oligonucleotides were used to amplify a target of 120bp of the conserved region of the DNA minicircle. The specific sequences of the primers were: hm1 – CCG CTA CCC CCC TTT TAC ACC AAC, MW=7504.8g/μmol; hm2 – GGG GAG GGG CGT TCT GCG AA, MW=6602.0g/μmol; and hm3 – GGC CCA CTA TAT TAC ACC AAC CCC, MW=7577.8g/μmol.14 DNA extraction was performed using the commercial Wizard Genomic Purification Kit® (Promega, USA), according to the manufacturer's instructions. The elution volume was 100μL. Each reaction included two negative controls and one positive control extracted from cultured promastigotes of L. infantum. The preparation of the amplification mixture was performed in an isolated environment, irradiated with UV light prior to mixing and free of DNA-amplified products or biological samples. The mixing was performed in a final volume of 10μL containing 1μL of the sample DNA, 0.2mM of dNTPs, 10mM of Tris–HCl (pH 8.6), 50mM of KCl, 1.5mM of MgCl2, 12pmol of each primer and 0.25U Taq polymerase (Promega, USA). The following amplification conditions were used: initial denaturation at 95°C for 5min, 39 cycles of denaturation at 95°C for 30s, annealing at 66°C for 30s, extension at 72°C for 30s and final extension at 72°C for 30s. PCR was performed using a Perkin Elmer GeneAmp PCR System 2400 (Perkin Elmer Corp., Norwalk, CT, USA). After amplification, 3μL of the sample was subjected to electrophoresis on a 6% polyacrylamide gel and stained with silver to visualize the amplified products.
Case definitionA positive result by any of the diagnostic tests administered defined an infected case.
Statistical analysisA database was constructed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA) containing data on subject identification, clinical examination, animal husbandry (pets and livestock), house and neighborhood characteristics, and results of the tests. The statistical analysis was performed using Pearson's chi-square test to compare the categorical variables. When indicated two-tailed Fisher's exact test was used. To compare means of variables with a normal distribution, Student's t test was used, and if these variables showed a non-Gaussian distribution the Mann–Whitney U test was used. The significance level was set at p<0.05. The relationship between infection with Leishmania (positivity to the LST) and the recorded data was explored with univariate logistic regression. A predictive model was selected to identify the variables associated with the risk of Leishmania infection detected by the Montenegro skin test positivity. The variables used were those that were biologically or epidemiologically plausibly associated with the outcome. Initially, we used a one-step univariate logistic regression, which enabled the selection of the variables that would be further evaluated in the multivariate analysis of the same type, in compliance with the criterion value of p<0.20. Prior to the entry of the variables in the multivariate analysis, a collinearity test was performed, and, subsequently, the interactions between the variables that showed statistical significance were assessed. All the tests were conducted with a 95% level of confidence.
Ethical issuesThe parents or legal guardians of individuals included in the study were informed of the possible risks of the study procedures, and those who agreed to participate signed an informed consent form. The project was approved by the Ethics Committee of the Faculty of Medicine of the University of Brasília (CEP-FM) – CEPFM 073/2006 process – on 28/03/2007. The study complied with the recommendations of the Helsinki Declaration and resolutions 196/1996 and 251/1997 from the National Board of Health on research involving humans.
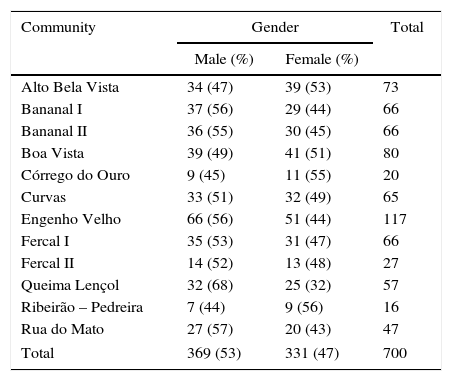
ResultsSeven hundred subjects were included. The distribution of this sample by the specific community of residence is shown in Table 1. The participants’ age ranged from two to 14 years, with a mean of 8.63±3.17 years and a median of 9 years (Q1/4=6.0 and Q3/4=11.0). Most of the evaluated subjects (590/700, 84.3%) were born in the Federal District. The average length of residence in the area was 7.38±3.5 years, with a median of 7 years (Q1/4=5.0 and Q3/4=10.0). Of the children and adolescents evaluated, 104 (14.9%) had previously lived in districts with transmission of VL, as determined by the data notifications of the Ministry of Health at the time of the study. Most of the participants (56/104, 53.8%) had lived in endemic areas of the northeast region, followed by 37 (35.6%) who reported having lived in the state of Goiás. In 85 (12.1%) cases, at least one dog had been collected by staff of environmental surveillance agency for suspected VL. Twelve subjects (1.7%) reported at least one case of VL in the family. No children or teenagers with fever, hepatomegaly, splenomegaly, or pallor were found. During the study (2007–2008), no individuals were classified as a suspected case of VL, and they were all classified as asymptomatic (n=700). One participant had a skin lesion, which was not compatible with cutaneous leishmaniasis.
Study sample distribution according to gender and communities of residence.
| Community | Gender | Total | |
|---|---|---|---|
| Male (%) | Female (%) | ||
| Alto Bela Vista | 34 (47) | 39 (53) | 73 |
| Bananal I | 37 (56) | 29 (44) | 66 |
| Bananal II | 36 (55) | 30 (45) | 66 |
| Boa Vista | 39 (49) | 41 (51) | 80 |
| Córrego do Ouro | 9 (45) | 11 (55) | 20 |
| Curvas | 33 (51) | 32 (49) | 65 |
| Engenho Velho | 66 (56) | 51 (44) | 117 |
| Fercal I | 35 (53) | 31 (47) | 66 |
| Fercal II | 14 (52) | 13 (48) | 27 |
| Queima Lençol | 32 (68) | 25 (32) | 57 |
| Ribeirão – Pedreira | 7 (44) | 9 (56) | 16 |
| Rua do Mato | 27 (57) | 20 (43) | 47 |
| Total | 369 (53) | 331 (47) | 700 |
Regarding the characteristics of the dwellings, 10 years was the median age of the houses (Q1/4=5.0 and Q3/4=13.0). Most of the houses were constructed of masonry (92.9%), and a few houses were wooden (6.7%). This condition occurred in all the communities, with the exception of Queima Lençol, in which 26.3% of the dwellings were built of wood. All of the assessed households had access to services providing electricity and water supply. Public sewer services were found in only one area of the Fercal I community and in two houses of Queima Lençol (which drained to the sewer system of the local school). The number of residents per home ranged from two to 13 people, with a median number of four people (Q1/4=4.0 and Q3/4=6.0). In all of the houses, the presence of annoying insects inside the home was reported. In response to questioning, 59 (8.4%) of the parents and caregivers reported that they protected their children using mosquito nets in the bedroom, and knowledge regarding the use of mosquito nets impregnated with insecticides was reported by the population. In the previous year, insecticide had been sprayed by health workers in 37 (5.3%) homes of the evaluated children and adolescents. Some spraying had been performed in houses with seropositive dogs after a canine serological survey. A minority of the spraying was performed due to convenience. At least one dog lived in 305 (43.6%) of the dwellings. This number ranged from zero to seven dogs per household. The overall average was 0.7±1dog/house. When only the houses with the presence of dogs were evaluated, that value increased to 1.61±0.92dogs/house, with a median of one dog/house (Q1/4=1 and Q3/4=2). Of the evaluated dogs, the predominant breed was mongrel, which was found in 387 (90.4%) of the cases. The dogs had a median age of two years. Male dogs were more numerous than females (248 vs. 180). The dogs were found predominantly in the Federal District, with a total of 414 (96.7%) dogs, and more than a half of them were born in the community of residence. The participants in 316 (45.1%) households reported keeping animals other than dogs. The most frequently reported animals were chickens (27.9%), other birds (14.6%), cats (13.6%), pigs (5.6%), and cattle (3.3%). In 225 dwellings (32.1%), the parents or guardians of the individuals assessed reported the presence of mammals in the vicinity. As the communities were more distant from the urban area of Sobradinho II, the presence of wild animals became more frequent. The presence of opossums (28.9%), monkeys (5.7%), foxes (1.7%), and armadillos (1.4%) was reported. This study did not define the specie of opossum. In the Fercal area the prevalent species is Didelphis albiventris.
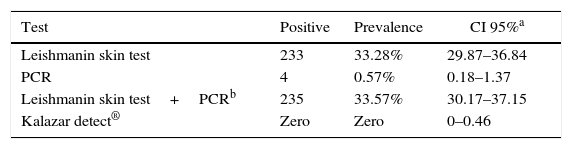
The diagnostic tests results are shown in Table 2. The prevalence of infection, as detected by a positive leishmanin skin test, was not homogeneous in all of the communities, ranging from 19.2% (Alto Bela Vista) to 53.8% (Boa Vista), with the highest values being found in the communities most remote from the urban area of Sobradinho. The positivity rate was higher among participants aged eight to fourteen years (39.7%) compared to the group of children up to seven years of age (22.5%) (X2=21.86, p<0.001). Among the four children with a positive PCR test, two were positive and two were negative for LST.
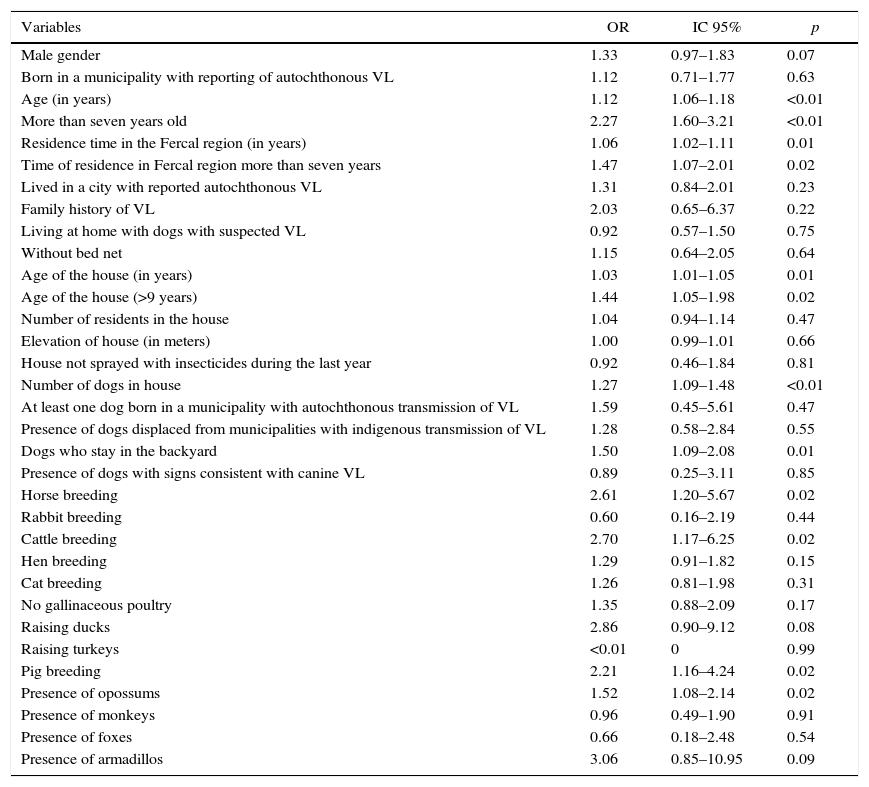
The statistical analysis of the factors associated with the outcome was performed using the results of the LST because of the low positivity of the applied serological and molecular tests. The univariate analysis showed that the predominant associated factor was the presence of domestic or wild animals in or around the houses, as shown in Table 3.
Univariate analysis of factors associated with Leishmania infection in Fercal, Brasília – DF.
| Variables | OR | IC 95% | p |
|---|---|---|---|
| Male gender | 1.33 | 0.97–1.83 | 0.07 |
| Born in a municipality with reporting of autochthonous VL | 1.12 | 0.71–1.77 | 0.63 |
| Age (in years) | 1.12 | 1.06–1.18 | <0.01 |
| More than seven years old | 2.27 | 1.60–3.21 | <0.01 |
| Residence time in the Fercal region (in years) | 1.06 | 1.02–1.11 | 0.01 |
| Time of residence in Fercal region more than seven years | 1.47 | 1.07–2.01 | 0.02 |
| Lived in a city with reported autochthonous VL | 1.31 | 0.84–2.01 | 0.23 |
| Family history of VL | 2.03 | 0.65–6.37 | 0.22 |
| Living at home with dogs with suspected VL | 0.92 | 0.57–1.50 | 0.75 |
| Without bed net | 1.15 | 0.64–2.05 | 0.64 |
| Age of the house (in years) | 1.03 | 1.01–1.05 | 0.01 |
| Age of the house (>9 years) | 1.44 | 1.05–1.98 | 0.02 |
| Number of residents in the house | 1.04 | 0.94–1.14 | 0.47 |
| Elevation of house (in meters) | 1.00 | 0.99–1.01 | 0.66 |
| House not sprayed with insecticides during the last year | 0.92 | 0.46–1.84 | 0.81 |
| Number of dogs in house | 1.27 | 1.09–1.48 | <0.01 |
| At least one dog born in a municipality with autochthonous transmission of VL | 1.59 | 0.45–5.61 | 0.47 |
| Presence of dogs displaced from municipalities with indigenous transmission of VL | 1.28 | 0.58–2.84 | 0.55 |
| Dogs who stay in the backyard | 1.50 | 1.09–2.08 | 0.01 |
| Presence of dogs with signs consistent with canine VL | 0.89 | 0.25–3.11 | 0.85 |
| Horse breeding | 2.61 | 1.20–5.67 | 0.02 |
| Rabbit breeding | 0.60 | 0.16–2.19 | 0.44 |
| Cattle breeding | 2.70 | 1.17–6.25 | 0.02 |
| Hen breeding | 1.29 | 0.91–1.82 | 0.15 |
| Cat breeding | 1.26 | 0.81–1.98 | 0.31 |
| No gallinaceous poultry | 1.35 | 0.88–2.09 | 0.17 |
| Raising ducks | 2.86 | 0.90–9.12 | 0.08 |
| Raising turkeys | <0.01 | 0 | 0.99 |
| Pig breeding | 2.21 | 1.16–4.24 | 0.02 |
| Presence of opossums | 1.52 | 1.08–2.14 | 0.02 |
| Presence of monkeys | 0.96 | 0.49–1.90 | 0.91 |
| Presence of foxes | 0.66 | 0.18–2.48 | 0.54 |
| Presence of armadillos | 3.06 | 0.85–10.95 | 0.09 |
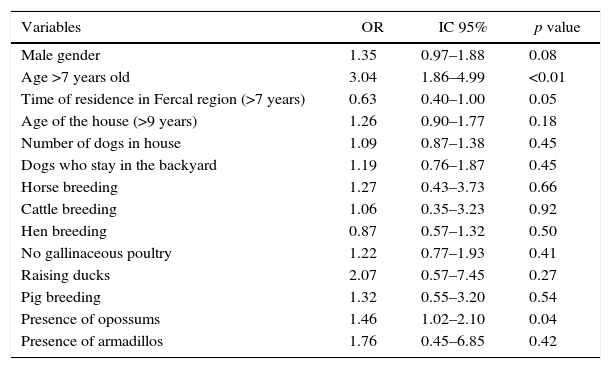
Fourteen variables were subjected to multivariate analysis after evaluation according to the Menard and Myers criteria of collinearity. The final result showed that human infection defined by positive LST was associated with age greater than seven years (OR=3.04, 95% CI 1.86–4.99) and with presence of opossums around the houses (OR=1.46, 95% CI 1.02–2.10). The other variables were not independently associated with the outcome, as seen in Table 4.
Multivariate logistic regression analysis of variables associated with Leishmania infection in Fercal, Brasília – DF.
| Variables | OR | IC 95% | p value |
|---|---|---|---|
| Male gender | 1.35 | 0.97–1.88 | 0.08 |
| Age >7 years old | 3.04 | 1.86–4.99 | <0.01 |
| Time of residence in Fercal region (>7 years) | 0.63 | 0.40–1.00 | 0.05 |
| Age of the house (>9 years) | 1.26 | 0.90–1.77 | 0.18 |
| Number of dogs in house | 1.09 | 0.87–1.38 | 0.45 |
| Dogs who stay in the backyard | 1.19 | 0.76–1.87 | 0.45 |
| Horse breeding | 1.27 | 0.43–3.73 | 0.66 |
| Cattle breeding | 1.06 | 0.35–3.23 | 0.92 |
| Hen breeding | 0.87 | 0.57–1.32 | 0.50 |
| No gallinaceous poultry | 1.22 | 0.77–1.93 | 0.41 |
| Raising ducks | 2.07 | 0.57–7.45 | 0.27 |
| Pig breeding | 1.32 | 0.55–3.20 | 0.54 |
| Presence of opossums | 1.46 | 1.02–2.10 | 0.04 |
| Presence of armadillos | 1.76 | 0.45–6.85 | 0.42 |
The study demonstrated the existence of asymptomatic infection by Leishmania spp. in the Fercal area. The magnitude of asymptomatic infection in the study area was greater than expected (33.28%). Some of the studied communities had a prevalence similar to endemic regions in which transmission has been demonstrated for decades. Few studies have investigated the extent of infection in areas with recent VL cases. The prevalence of infection was similar to that reported by Cunha et al., who evaluated asymptomatic children and adults in an area of recent introduction of VL in the state of Bahia.15 In the city of Natal, Northeast Brazil, five years after the report of the first case of human VL, Jerome et al. reported a 38% positivity of the neighbors and cohabitants of people with VL.16 In the city of Teresina, also in Northeast Brazil, a survey using identical methodology to that used in this study, Gouvea et al. described a prevalence of infection of 36.7%.8
Fercal is a recently settled territory; therefore, the decision to select children and adolescents for the study avoided bias related to a participant having previously lived in areas of leishmaniasis transmission. Because a leishmanin test could remain positive after many years of contact with endemic areas, the selection of young people helps to reduce that bias, due to decreased exposure time. The leishmanin test which was selected to define the state of Leishmania infection has the following advantages: it is inexpensive; it is simple to apply and read; no equipment or laboratory facilities are required for the application, reading and interpretation; and it enables the measurement of infection by Leishmania species causing VL in areas without the presence of cutaneous leishmaniasis (CL). Some studies have suggested that people with a negative leishmanin test had either no contact with the parasite or have had contact without developing reactivity to the test. The latter are individuals more likely to become ill with VL.17 The Fercal area has sporadic cases of CL, and there are no reports of the mucosal form of leishmaniasis. From 2000 to 2008, 307 cases of CL were reported in Brasília, and among them, two cases were considered autochthonous from the Fercal region.18 In populations that have had recent or sporadic cases of CL, the prevalence of asymptomatic infection identified by a positive LST has been shown to range between zero and 3.8%19; in areas of high endemicity including Corte de Pedra in the State of Bahia, the prevalence increases to 47%.20 These findings suggest that the positive results of this study reflect the magnitude of L. infantum infection.
The finding of zero prevalence when an immunochromatographic assay is used is in disagreement with previous studies of asymptomatic infection by Leishmania. This test has been used in Brazil for this purpose, and variable prevalence rates have been observed in studies performed. A study conducted in Minas Gerais showed that the rapid test had low positivity rates in population surveys, respectively 5.6% and 4.3% in Sabará4 and 4,3% in Porteirinha.6 In Montes Claros – MG, no positive cases were found among blood donors with positive serological tests for Leishmania.21 The molecular test used in this study had lower performance than those described previously. In Brazil, the positivity of this test has varied between 7.2% in Teresina (Piauí)22 and 29.7% in Belo Horizonte – MG.23 These studies were performed in relatives of people with VL, which could explain the higher positivity rate when compared with surveys in randomly selected asymptomatic individuals. The small parasitic load in asymptomatic patients may explain the low performance of our molecular tests.4 The finding that not all patients with a positive serologic test have positive DNA tests should not necessarily be interpreted as a lack of specificity, but as a demonstration of the rapid circulation of the parasite into the bloodstream, which could explain the association of a positive leishmanin test with asymptomatic infection. Positive molecular tests might be present in asymptomatic individuals with negative serology as well.22,24
The estimated prevalence (33.3%, 95% CI 29.9–36.8%) in the Fercal region was based on the results of the leishmanin test. This prevalence is in line with previous surveys in areas of recent introduction of the disease in Bahia and Rio Grande do Norte where positivity rates of 32% and 38%, respectively, were found.15,16 In areas of long-lasting VL transmission in Brazil, the prevalence has varied from 2.9 to 71%.22,23 We hypothesize that the prevalence identified in this study is a result recent introduction of by Leishmania species infection in the area, where there is widespread susceptibility of dwellers. This explains the asymptomatic infection of a large number of susceptible individuals, particularly those with no prior contact with endemic areas, such as children and adolescents born in the area of the recent introduction of the parasite.
Fourteen characteristics were potentially associated with the prevalence of Leishmania infection measured by LST. After the multivariate logistic regression analysis, the two variables that remained associated with the outcome were older age and presence of opossums in the neighborhood. Previous studies have described the association variable magnitude between age and asymptomatic VL. However, most of them agree that risk begins to increase before 10 years of age. In Italy, Biglino et al. studied Leishmania infection using Western blot analysis and reported that the age associated with the condition was over 65 years.25 Our study corroborates the findings of Badaró and colleagues, who described, in Jacobina city of Bahia, Brazil, that positivity for the leishmanin test begin to increase at the age of seven years.26 In the municipality of Raposa, in Maranhão, Brazil, Caldas et al. indicated 23 months as the cutoff age associated with the rise in prevalence, showing that children in endemic areas are infected at an early age. In another municipality in Maranhão, the cutoff age was five years, and 56.7% of children and adolescents were positive for the leishmanin test, compared to 35% of the children younger than five (p<0.001).11 In Teresina, in Piaui, Gouvea et al. reported an association of age with a positive leishmanin test; however, this association disappeared when the outcome was seropositivity.8 In an area of recent introduction of the disease in the state of Bahia, Cunha et al. found no association of infection with age or the assessed characteristics.15 Additionally, a study conducted in a low-endemicity area, Araçatuba, São Paulo by Barão et al. showed no association of VL with age.12 Regarding the presence of opossums in the assessed dwellings, this animal was reported most frequently. An open question was asked to assess this variable, and the question was intended to pertain only to mammals and to the presence of wild animals around the homes. The presence of opossums was reported in an even higher proportion than that of farm animals, and opossums were seen more frequently around houses than other animals, except for dogs. The role of this marsupial as a reservoir of LV has been shown,27,28 and some authors consider the species to be a potential reservoir in urban areas.28 Although the first references to its potential as a reservoir were in the early 1980s, in Brazil, its role has rarely been studied, and there are more frequent reports in Colombia and Venezuela.29–31 The opossum has been linked to the transmission of L. infantum chagasi and the species associated with CL.32,33 In Colombia, the opossum and a wild rodent were shown to be the best dietary sources of Lutzomyia evansi (the VL vector in northern Colombia).31 In Rio de Janeiro, this marsupial has been associated with canine VL.34 As reported in the study conducted in Belo Horizonte, the opossum could represent an important reservoir of VL, to be considered in the control measures for this infection.28 One study of cases of VL in Belo Horizonte from 1999 to 2000 showed that the presence of household animals was related to the occurrence of the disease and considered the possibility of marsupial – in addition to dogs, horses, cattle and pigs – involvement in VL transmission.35 A recent study performed in conservation units around Brasília found Didelphis alviventris with positive molecular tests for Leishmania, confirming the presence of this species as a potential reservoir and suggesting that a sylvatic cycle could be beginning to settle in this area.36 Complete understanding of the role of opossums in the epidemiology of leishmaniasis should provide appropriate information for control activities.
This study highlights the importance and distribution of asymptomatic infection by Leishmania in an area of recent introduction of VL. New studies that overcome the limitations faced in this research are warranted in order to check the consistency of our findings. This study suggests that opossums could be the link in the transmission of VL between rural and urban areas. The role played by opossums in attracting vectors from wild to urban areas should be further explored in future studies. We suggest that specific studies should be directed at understanding the role of synanthropic and domestic animals other than dogs in areas of recent transmission of VL. The investment in reducing the asymptomatic infection by L. infantum is meaningful considering that co-infection with Leishmania/HIV is increasing in Brazil, with a higher fatality rate; at least some of those co-infected individuals acquired Leishmania infection at an earlier phase of life.37
Conflicts of interestThe authors declare no conflicts of interest.
To Renata Ribero de Sousa, technician at the Laboratory of Leishmaniasis of the Tropical Medicine Center of the University of Brasília, for her valuable contribution to the laboratory work; to José Barbosa Bezerra, for his collaboration in the field work; and to the officers of the Epidemiological Surveillance Unit of the State Secretary of Health of Federal District (SES-DF) for their support during the field work.