In the present paper we summarize the suggestions of a multidisciplinary group including experts in pediatric oncology and infectious diseases who reviewed the medical literature to elaborate a consensus document (CD) for the diagnosis and clinical management of invasive fungal diseases (IFDs) in children with hematologic cancer and those who underwent hematopoietic stem-cell transplantation. All major multicenter studies designed to characterize the epidemiology of IFDs in children with cancer, as well as all randomized clinical trials addressing empirical and targeted antifungal therapy were reviewed. In the absence of randomized clinical trials, the best evidence available to support the recommendations were selected. Algorithms for early diagnosis and best clinical management of IFDs are also presented. This document summarizes practical recommendations that will certainly help pediatricians to best treat their patients suffering of invasive fungal diseases.
Invasive fungal diseases (IFDs) have become an important cause of morbidity and mortality in immunosuppressed patients, especially those with cancer and/or undergoing hematopoietic stem cell transplantation (HSCT).1,2
Pediatric patients are equally susceptible to IFD compared to the adult population. However, there are relevant differences in the physiology, presence of comorbidities, pharmacokinetic and tolerance to antifungals, treatment regimens, underlying diseases evolution, populations at risk and prognosis of fungal infections. Therefore, the extrapolation of clinical practices consolidated in the adult population for children is sometimes inappropriate, making it necessary to create specific guidelines for the clinical management of IFD optimized for pediatric patients assisted in our country. In order to propose algorithms for early diagnosis and best clinical management of IFDs in pediatric patients we reviewed all major multicenter studies designed to characterize the epidemiology of IFDs in children with cancer, as well as all randomized clinical trials addressing empirical and targeted antifungal therapy. In the absence of randomized clinical trials, the best evidence available to support the recommendations were selected.
Epidemiology of invasive fungal diseases in cancerThe incidence of IFDs in the pediatric population with cancer and/or undergoing allogeneic HSCT is highly influenced by the use of prophylactic systemic antifungal agents, the availability of tests and procedures for earlier diagnoses, the adopted IFD definitions, population denominators, and local epidemiology.1,2
The pediatric patients at greatest risk for developing IFD are children undergoing allogeneic HSCT, those with acute myeloid leukemia (AML) and cases of relapsed acute lymphoblastic leukemia (ALL).3 Children with acute leukemias are continuously exposed to multiple risk factors for IFD and up to one third of them may develop IFD in the absence of antifungal prophylaxis.4–7
IFDs after allogeneic HSCT have a bimodal pattern of incidence that is secondary to the presence of different risk factors over time after transplantation. The first phase of risk is basically related to the status of the underlying disease, the immunosuppression secondary to the conditioning regimen, intensity and duration of neutropenia, and mucositis that take place before stem cell engraftment. Pending on the intensity and duration of neutropenia, patients may exhibit susceptibility to yeasts or also molds. After the engraftment and recovery of neutrophil count, the risk of fungal infections is mostly dependent of the presence of acute and chronic graft-versus-host disease (GvHD). As expected, the use of antifungal prophylaxis and the use of high efficiency particulate air filters (HEPA) substantially decrease the risk of fungal infections.2,8
Mortality due to IFDs varies from 20% to 70%, depending on the intensity of the immunosuppression, presence of comorbidities, the availability of tools for early diagnosis, the site and severity of the infection, as well as the time to initiate therapy and antifungal regimens used. Lower survival rate is usually seen in patients with disseminated fungal disease, central nervous system (CNS) involvement, underlying disease refractory to the first regimen of chemotherapy and persistent neutropenia.8,9 During periods of deeper immunosuppression, use of antifungal prophylaxis may mitigate the risk of acquiring IFD, reducing morbidity and high mortality rates of these infections.2,10
It is important to consider that costs of antifungal treatment are always a matter of concern by health care policy makers. However, economic analyses in health care should consider not only the cost of different antifungal regimens, but also their impact in terms of reducing length of hospitalizations and intensive care admissions, toxicity, use of blood products, and delays for delivering appropriate antineoplastic therapies, which may hamper the chances of curing these children.11–13
Variables that may modulate the risk of IFDs in cancer patientsAgeAge is an independent risk factor for IFDs in children being treated for AML or undergoing HSCT.8,14 Younger children may have less effective phagocytic activity and T-lymphocyte recovery after being exposed to intensive chemotherapy.7,15–17 The differences are more significant when comparing immune responses in extreme pediatric ages. Children with AML tend to be older, and it may also contribute to their incidence of IFDs. In addition, older children and adolescents are more susceptible to mucous membrane injury due to chemotherapy exposure, and mucositis may also increase the risk of IFDs.14
NeutropeniaThe risk of IFDs is directly related to the intensity and duration of the neutropenia. Chemotherapy further impairs neutrophil, macrophage, B and T-lymphocyte function, also decreasing immunoglobulin production and opsonization.5,14,18
A neutrophil count lower than 500/mm³ for longer than 10 days is an important risk factor for IFDs, but the risk is highest with counts below 100/mm³ in patients with malignant neoplasms, on corticosteroids and chemotherapy,10 AML induction, and early post HSCT. A low lymphocyte count of less than 100/mm3 further increases the incidence of IFDs in neutropenic febrile children after chemotherapy.19 The use of some new immunosuppressors, as infliximab, may be, by itself, a risk factor for invasive aspergillosis.20
Host impact of anti-neoplastic treatmentThe intensity of the chemotherapy regimen in pediatric oncology is driven by many different factors, such as proliferative index of the tumor, stage and phase of the disease, risk of relapse, and the dose-limiting toxicities of the different chemotherapy combinations. Children with AML, relapsed or refractory acute leukemias (both lymphoid and myeloid), and undergoing HSCT have the highest risk of developing IFDs. Once these high-risk patients are identified, they should be maintained under protective environment, including rooms with HEPA filter, clean water and diet free of food-borne fungal contamination. In addition, they should be submitted to an intensive workup to actively screen for IFDs, and different regimens of antifungal prophylaxis should be also considered.5–7,10,14,21,22 The treating team must be aware that these children are also at a very high risk of bacterial and viral infections.22
In ALL patients, steroids used during induction therapy amplify the negative impact of neutropenia by impairing phagocytosis, neutrophil migration and humoral immune response. The use of an equivalent prednisone dose of 2 mg/kg/day is associated with a high risk of IFDs.3,8 High-dose antimetabolites, anthracyclines and intensive asparaginase regimens lead to rupture of the mucosal barriers in the gastrointestinal tract, further increasing the occurrence of IFDs.3,5,17,22 In patients undergoing allogeneic HSCT, the use of high-dose steroids for over 10 days and its prolonged use to treat GvHD are additional risk factors for IFD.8
Even minimizing invasive procedures during treatment, such as bone marrow aspirates, cerebrospinal fluid collections, peripheral venipunctures and insertion of central venous catheters, children continue susceptible to bloodstream invasion by translocation of endogenous microbiota. All efforts should be taken to warrant that health workers working with hematologic patients will be committed with best practices for controlling infections related to health assistance.8,10
Impact of broad-spectrum antibioticsThe overuse of broad-spectrum antibiotics for empirical treatment of febrile neutropenia changes the endogenous microbiota, favoring colonization by fungal pathogens. Indeed, the use of antibiotics for more than seven days is associated with an increased risk of IFDs.6
Impact of candida antifungal prophylaxisThe prolonged use of prophylactic fluconazole, often indicated in high-risk patients, substantially decreases the incidence of candidemia, usually to less than 1%, but it may contribute to a selection of fluconazole-resistant fungal pathogens such as C. glabrata and C. krusei.8
Impact of diseases requiring HSCTThe underlying diagnoses of severe aplastic anemia, including Fanconi anemia,12 and relapsed or refractory leukemia21 significantly increase the chance of developing IFDs after HSCT. Prolonged length of neutropenia and the aggressive chemotherapy received by these patients may further compound the risk for IFDs.
Impact of specific HSCT-related treatment strategiesReduced intensity and non-myeloablative conditioning regimens have a lower risk of IFDs when compared to conventional myeloablative regimens during the pre-engraftment period. Bone marrow and umbilical cord blood as graft sources have higher risk of IFDs than peripheral blood stem cells.8,11 T-cell depletion of the marrow or peripheral blood grafts further increases the risk of all opportunistic infections, including IFDs.23
The immunosuppressive agents selected to prevent and treat GvHD represent a major risk factor for IFD in transplanted patients after engraftment.9,21,24 The presence of cytomegalovirus (CMV) reactivation may have an immunosuppressive effect that can increase patients’ susceptibility to IFDs. This mechanism of immunosuppression has not been completely characterized, but it is not only secondary to ganciclovir-induced neutropenia. In fact, it is well established that CMV has a negative immunomodulatory effect on the activity of neutrophils and macrophages.8,25–27 A significant increase in the risk of fungal infection is observed when the patient is CMV-seropositive, compared with both patient and donor with CMV-negative serology (p < 0.01).26
Protective environmentHand washing is an important part of preventing fungal diseases. Candida parapsilosis infections have been associated with the exposure of patients to central venous catheterization and the use of parenteral nutrition. In this scenario, the transmission of this agent can occur through the hands of healthcare providers, since these organisms are frequently present in their hands.8
It is very important to minimize exposure to airborne fungal propagules throughout hospitalization, not only during the period of neutropenia.8 A high efficiency particulate air (HEPA) filter, providing an air filtration rate of more than 12 exchanges per hour, is highly recommended in hospital facilities assisting allogeneic HSCT recipients and AML patients in order to decrease their risk for developing infections due to airborne filamentous fungi.28
Etiology of invasive fungal infections in pediatric cancer patients and their clinical impactCandida spp continues to be responsible for the majority of IFDs among onco-hematologic pediatric patients. However, in the past decades, the number of patients infected by filamentous fungi has increased, including Aspergillus spp, Mucorales, and Fusarium spp.22,29–31
This new epidemiological scenario is probably related to the widespread use of prophylaxis with fluconazole, the intensity of immunosuppression secondary to new chemotherapy regimens and the development of better diagnostic tools for fungal infections.1,21,32
The clinical presentation of candidemia is usually fever or sepsis refractory to broad spectrum antibiotics. C. albicans, C. parapsilosis and C. tropicalis are the most frequent isolates. Disseminated candidemia is reported in 10–20% of the pediatric patients; severe sepsis and septic shock occurs in 30%. Mortality rates vary between 10% and 25% and can be up to 50% in patients admitted to an intensive care unit.1,33,34
In the HSCT setting, Candida spp infections are usually identified within the first month after transplantation, especially in patients with severe mucositis, during neutropenia or immediately after neutrophil recovery. Other IFDs can also occur in this period, but they are usually due to airway colonization and immunosuppression prior to the transplant.21,32
Epidemiological studies of invasive Aspergillosis (IA) in pediatrics are usually represented by single center reports.1,4,8,35,36 The prevalence of aspergillosis in hematologic pediatric patients range around 5–10% in patients with AML, relapsed or refractory leukemias, and post allogeneic HSCT. At St Jude Hospital (Memphis, USA), the highest incidence was found in patients with MDS (8%).35 The lethality rate ranges from 20% to 50%, but it is up to 80% after allogeneic HSCT.4
A retrospective study of 485 pediatric HSCT patients at Duke University (USA) reported an incidence of IA of 5%, with a higher incidence among allogeneic HSCT recipients36; similar to the findings of two others databases from the USA.11,14
The first peak in the incidence of IA occurs between D + 15 and D + 30 after HSCT because of prolonged neutropenia. A second peak takes place between D + 60 and D + 180 and is associated with chronic GvHD and with the use of immunosuppressive drugs, especially corticosteroids.1,8,32,34,21,38 Fungal infections in the early period after transplantation are usually documented in scenarios of HLA incompatibility, intense myeloablative conditioning regimens as well as the lack of protective environment for assisting the patients. Late fungal infections are usually documented in the presence of GvHD, exposition to high steroid doses and in patients with CMV reactivation. A previous history of mold infections may also increase the risk of IFD after HSCT.1,8,32,34,21,38
Similar to adults, most pediatric patients present with pulmonary aspergillosis. Dissemination to other sites, particularly the CNS, may occur in up to 30% of the cases with a late diagnosis.39 Of note, cutaneous presentation of IA appears to be more common in children than in adults, probably due to traumatic lesions followed by direct inoculation of fungi or because of fungemia. The skin lesions initially present as non-specific erythematous or edematous plaques, sometimes very painful, may progress to necrotic ulcers. Patients at risk of fungal infection with skin lesions must undergo biopsy and culture of the lesions.36
IFDs caused by filamentous fungi other than Aspergillus, i.e., Fusarium spp, Scedosporium spp and agents of mucormycosis may have a clinical presentation very similar to aspergillosis. In our country, special attention must be paid to infections caused by Fusarium spp. Fusarium may be present in the pre-transplant period as apparently innocent paronychia that may further progress to fast bloodstream invasion and sepsis if superficial infection were left untreated along the period of immunosuppression of the patient. In a recent Brazilian multicenter epidemiological study fusariosis was one of the most common IFD documented among high-risk hematologic patients, including cases of HSCT, MDS and AML.29 Patients usually develop fungemia, painful skin papules surrounded by an erythematous halo and central necrosis, and pneumonia. Serum galactomannan is often positive, increasing the confusion with IA.40,41
The literature of invasive fusariosis is mostly related to adult patients. However, an ever increasing number of cases of invasive fusariosis has also been reported in pediatric patients.42,43
Diagnostic tools in clinical mycologyConventional methods for the diagnosis of IFDsThe diagnosis of IFD in patients with malignant hematologic disease and/or undergoing HSCT should take into consideration the presence of specific risk factors, as well as clinical, radiological, and microbiological findings. To optimize the sensitivity and accuracy of the diagnosis of fungal infections, molecular tests (PCR based methods and assays for detecting fungal antigens) should be used in combination to conventional methods, as well as direct examination, histopathology, and culture.44,45
Direct examinationDirect microscopic examination of clinical materials is one of the most straightforward methods, simple and useful for the diagnosis of fungal infection of the respiratory tract and skin lesions, as reported in patients with fusariosis.46 Advantages of this method include the low cost and short turn-around time for providing results, but it may have a sensitivity lower than 50%, especially in patients already receiving systemic antifungal agents.44
CultureCultures play a fundamental role in the diagnosis of fungal infections, providing an accurate characterization of the agent. However, sensitivity is low (<50%) and final results may take many days pending on the pathogen. The volume of the biologic sample may substantially impact the sensitivity of the test what should be considered along the collection of the samples according with the bottle used (neonates <4 kg: 1 mL; pediatric: 4–13.9 kg: 3 mL, 14–24.9 kg: 10 ml and adults or >25 kg: 20 mL).47 Automated blood culture systems, such as Bactec™ (BD Instruments, USA) and BacT/Alert® (bioMérieux, Brazil) have sensitivity around 50% for invasive candidiasis.48
The identification of fungi at species level is mandatory for the adequate characterization of the IFD epidemiology in each center, as well as to allow the adequate selection of strategies for prophylaxis, control and treatment of the infection. The identification of filamentous fungi is usually based on the analysis of the micro-morphological characteristics of the reproductive mycelium of the mold pathogen isolated in culture. Accurate species identification of molds is highly dependent on the combination of data provided by colony micromorphology examination and sequencing of specific DNA target regions, a strategy that may allow discrimination of genetic closely related species. More recently, commercial systems based on proteomic analysis performed by Matrix Associated Laser Desorption-Ionization — Time of Flight (MALDI-TOF) have been integrated to the routine of clinical laboratories providing accurate automated identification of yeast and molds within few minutes.49–51
HistopathologyThe gold standard for the diagnostic of IFD caused by a mold is the histopathologic demonstration of tissue invasion by a filamentous fungi complemented by culture of the biologic sample with accurate identification of the pathogen. However, tissue biopsies are not frequently performed in hematologic patients due to the coexistence of coagulopathies, thrombocytopenia, neutropenia and clinical instabilities, such as respiratory failure. When available, the tissue sample should be cultured and sent for histopathological evaluation with hematoxylin and eosin (HE) and specific staining for fungi (periodic acid Shiff (PAS) and/or Grocottstain (methenamine-silver).44
Role of biomarkers for mycological diagnosisDuring episodes of IFDs, fungal antigens can be released into the bloodstream before the appearance of any clinical or radiological abnormalities in a substantial number of patients. Biological samples collected for the detection of fungal biomarkers in serum or bronchoalveolar lavage may provide an earlier diagnosis of IFD in patients with malignant hematological diseases and/or undergoing HSCT.44,52
Detection of galactomannan (GM) in biological fluidsGM is a polysaccharide present in large amounts in the cell wall of the Aspergillus spp. It is released into infected host tissues during the hyphae growth. Despite its relevance for the diagnosis of invasive aspergillosis, this antigen is also present in other fungal agents and can generate positive results in infections caused by Fusarium sp and Histoplasma capsulatum, among others.53
In Brazil, the sandwich-type immunoenzymatic assay (ELISA, Platelia™ Aspergillus, RioRad, France) is commercially available for detecting this antigen. Final results may be obtained after approximately three hours, much faster than tissue culture. Galactomannan detection in biologic samples from high-risk patients has been used with great success to aid in the early diagnosis of IA.54
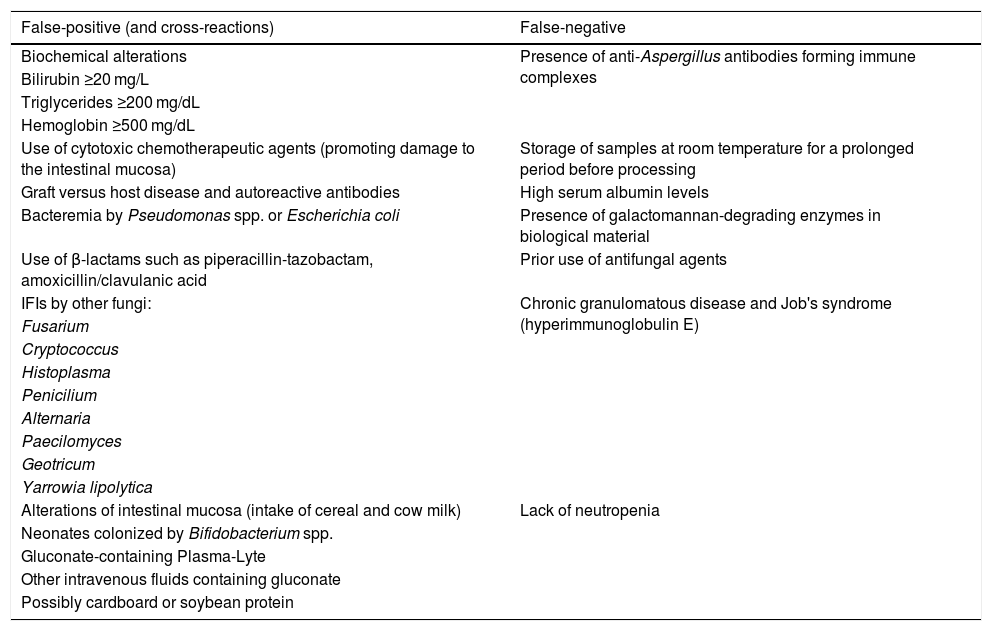
In adults, in a significant number of patients, circulating GM may be detected at a median of five to eight days before clinical and radiological manifestations of aspergillosis. Based on several studies, GM positivity in serum, bronchoalveolar lavage fluid, or cerebrospinal fluid has been accepted as mycological criteria of IA in the revised European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and by the National Institute of Allergy and Infectious Diseases Mycoses Study Group — EORTC/MSG.55 False-positive and false-negative results can occur for several reasons (Table 1).53 Lower sensitivity rates are documented in non-neutropenic patients, without onco-hematological diseases, as well as in patients on anti-mold antifungal prophylaxis.57,58
Factors that can lead to false-positive and false-negative results with the galactomannan test.63
| False-positive (and cross-reactions) | False-negative |
|---|---|
| Biochemical alterations | Presence of anti-Aspergillus antibodies forming immune complexes |
| Bilirubin ≥20 mg/L | |
| Triglycerides ≥200 mg/dL | |
| Hemoglobin ≥500 mg/dL | |
| Use of cytotoxic chemotherapeutic agents (promoting damage to the intestinal mucosa) | Storage of samples at room temperature for a prolonged period before processing |
| Graft versus host disease and autoreactive antibodies | High serum albumin levels |
| Bacteremia by Pseudomonas spp. or Escherichia coli | Presence of galactomannan-degrading enzymes in biological material |
| Use of β-lactams such as piperacillin-tazobactam, amoxicillin/clavulanic acid | Prior use of antifungal agents |
| IFIs by other fungi: | Chronic granulomatous disease and Job's syndrome (hyperimmunoglobulin E) |
| Fusarium | |
| Cryptococcus | |
| Histoplasma | |
| Penicilium | |
| Alternaria | |
| Paecilomyces | |
| Geotricum | |
| Yarrowia lipolytica | |
| Alterations of intestinal mucosa (intake of cereal and cow milk) | Lack of neutropenia |
| Neonates colonized by Bifidobacterium spp. | |
| Gluconate-containing Plasma-Lyte | |
| Other intravenous fluids containing gluconate | |
| Possibly cardboard or soybean protein |
A recent systematic review addressing the accuracy of GM in pediatric patients analyzed results of 10 studies in which GM was used for screening of fungal infections in high-risk patients and eight studies evaluating GM as a diagnostic tool in the presence of specific clinical/radiological findings. Excluding cases of possible IFD, only five studies for each one of the settings (screening versus diagnosis) remained in the pooled analysis of specificity and sensitivity. In the cohort where GM assay was used for screening IA, specificity and sensitivity values were 91% [95% confidence interval (CI): 86–94%] and 68% (95% CI: 51–81%), respectively. Otherwise, in the setting where GM was requested only at the moment of diagnosis, specificity and sensitivity values were 85% (95% CI: 51–97%) and 89% (95% CI: 79–95%), respectively.59
The positive predictive values (PPVs) in children were rather low and ranged between 0 and 100% in each setting, while the negative predictive values (NPVs) were considerably higher, ranging from 85 to 100% and from 70 to 100% in the screening and diagnostic setting, respectively.59
Data on GM in pediatric patients are still controversial to support a general recommendations for the clinical use of this biomarker for screening of aspergillosis in high-risk patients.60 Some medical centers started to use serum GM monitorization of high-risk patients as part of their strategy for preemptive treatment of aspergillosis.61 Experience with GM screening of pediatric patients is still scarce to provide any general recommendation.
Galactommanan test positivityTwo consecutive serum samples exhibiting galactomannan index ≥0.5 or one result ≥0.7 are suggestive of IA. For bronchoalveolar lavage (BAL) samples, the cutoff it is still controversial: the manufacturer considers an index ≥0.5 in the BAL as positive, but most meta-analyses and pediatric studies suggest that a positive result requires GM index ≥0.8 or 1.0 in the BAL.1,51,52,53
1,3 β-d-glucan (BDG)BDG is a polysaccharide found in large quantities in the cell wall of several pathogenic fungi including Candida spp., Fusarium spp, Aspergillus spp, and agents of endemic mycoses. The presence of BDG in blood or other biological fluids can be a serological marker of fungal sepsis in patients with candidemia, aspergillosis, fusariosis, or infections caused by Pneumocystis jiroveci, Acremonium spp. or Saccharomyces spp. It is important to mention that this test is negative in infections caused by Cryptococcus and all Mucorales.44,63
A limitation of this test is the large number of conditions that may generate false-positive results and the small number of studies addressing the relevance of this test in pediatric populations.59,64
In Brazil, ANVISA approved the Fungitell® commercial test (Associates of Cape Cod, Inc.) for clinical use. In adults, results below 60 pg/ml are considered negative, values between 60−80 pg/ml inconclusive (suggesting the repetition in a new sample) and >80 pg/ml is considered positive. In patients at risk of IFD, different authors recommend serum collection twice a week.
Recently the Dynamiker Fungus (1-3)-B-d-Glucan Assay (Dinamiker Biotechnology (Tianjin) CO., LTD, Popular Republic of China) has been approved by ANVISA for IFI diagnosis. Although its performance is equivalent to Fungitel, it is seldom used in Brazil.65
Cutoff values of (1-3)-B-d-Glucan commercial tests for diagnosing fungal infections in pediatric patients are not yet well validated.64,66
Using images as a diagnostic toolImaging tests are useful tools for evaluating patients with suspected IFD, especially with involvement of the respiratory tract and central nervous system. Due to limitations in sensitivity of conventional chest X-ray (CXR) to detect IFD in immunocompromised patients, CT scans are strongly recommended for screening patients at risk.
Prospective studies conducted in adult patients have demonstrated that CT scans can detect pneumonia earlier than CXR. Indeed, when CT scan is systematically performed in patients at high risk for IFD it may provide earlier diagnosis of invasive pulmonary aspergillosis with improvement in prognosis.67,68
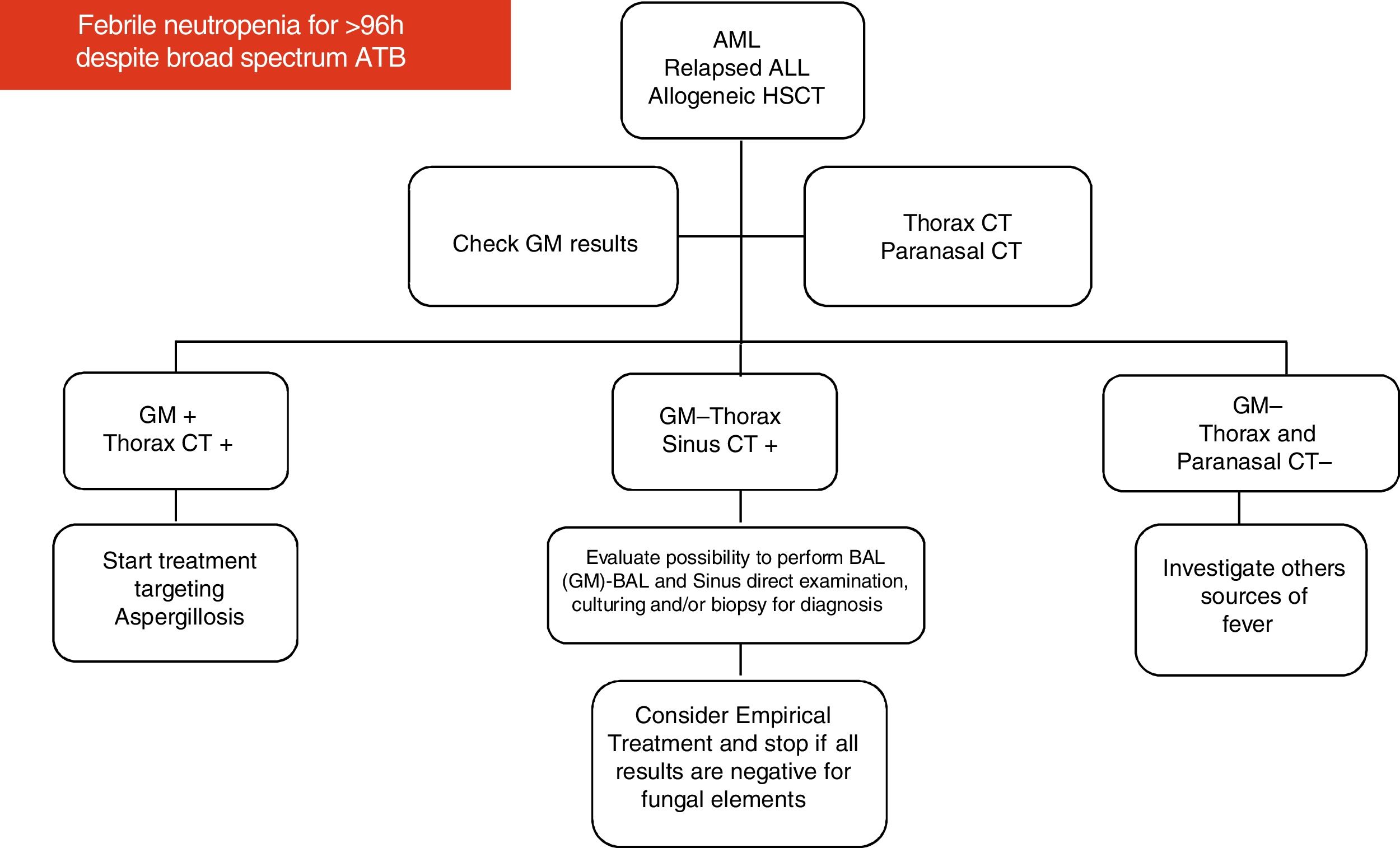
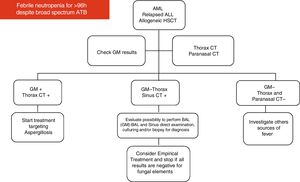
Imaging for investigation of neutropenic patients with persistent feverAccording to the 2017 guidelines on clinical management of children with febrile neutropenia, imaging studies, including chest CT scan or adequate imaging of the symptomatic organ/system, should be performed in the evaluation of children considered to be at high-risk for developing IFD, who remain febrile and neutropenic for more than 96 h despite adequate antibiotic coverage.10,60
There is little data on the evaluation of children suspected of sinonasal IFD. Notably, children with less than two years of age do not have sufficient pneumatization of the sinus cavities, and thus, sinus imaging is rarely informative in this age range. The role of routine abdominal imaging is uncertain in patients with persistent febrile neutropenia, and imaging of abdominal lesions may generate falsely negative results.60
Imaging for checking patients with pulmonary infectionsImaging findings in infants with pulmonary involvement can differ from those in older children and adults.
Candida involvement of the lungs is rare in this setting of patients. The few cases reported in this population are typically secondary to hematogenous dissemination and lung lesions are usually represented by infarctions, homogeneous consolidations such as bacterial lobar pneumonia or presence of mass-like rounded images with or without central necrosis, as occur with Aspergillus.69
The image spectrum of pulmonary aspergilosis may include ground-glass appearance, small nodules, larger nodules to segmental or lobar consolidations, and invasion of the rib cage. Hilar lymph node enlargement and small pleural effusions may occur.70
The presence of small nodules or consolidations with surrounding ground-glass area, known as the halo sign, resulting from perilesional hemorrhage, secondary to angio-invasion, suggests angio-invasive aspergillosis in the neutropenic patient. The halo sign is most commonly reported in pulmonary aspergillosis but is not pathognomonic of this fungal infection and may occur secondary to other conditions. Cavitation and air crescent formation might occur more frequently in older children and adults with aspergillosis, when compared to younger children.68,71,72
The largest series reporting aspergillosis in children was collect by Burgos et al. in 2008, addressing 188 pulmonary radiographic findings generated with 110 patients with pulmonary disease where nodules were documented in 59% of the cases. Nodules were less frequently seen in the youngest children compared to the older age groups: nodules were seen in 38.7% (12 of 31) of the 0- to 5-year-olds compared with 71.8% (28 of 39) and 62.5% (25 of 40) of the 6- to 12-year-old and 13-year-old groups, respectively. Only three (2.2%) of 110 patients showed the air crescent sign, and none of them was reported within the group with 0- to 5-year-olds.4,72
In terms of obtaining accurate diagnosis of fungal infections, it is mandatory to correlate radiological findings with clinical presentation, the host immunity status, results of fungal biomarkers, and data provided by conventional microbiology assays.10,44
There is currently great concern regarding the radiation dose of the radiological exams, as these patients undergo multiple examinations throughout the follow-up period.
Chronic disseminated candidiasis or hepatosplenic candidiasis (CDC)CDC is a persistent Candida infection of the liver, spleen, and others organs that may follow candidemia in neutropenic patients after recovery of neutropenia.73 When restricted to the liver or spleen, this entity is often referred to as hepatosplenic candidiasis (HSC).44
Ultrasonography (USG) remains a useful tool for detecting and monitoring CDC.74 The main advantage of this method is the lack of nephrotoxicity and radiation exposure to perform the exam, but its main limitation is that USG might not detect small lesions. The use of contrast with USG might improve the yield of USG for detecting fungal lesions that are represented by disseminated hypoecogenic small lesions. A typical finding of CDC lesions in the liver is the bull’s eye or target pattern with peripheral hypoecogenic halo encircling a central hyperechogenic core.73
MRI seems to be superior to CT in identifying liver lesions associated with CDC; its sensitivity is 100% and specificity is 96% when the appropriate techniques are used.75,76 The major drawback of MRI use is cost and availability. In this regard, contrast-enhanced biphasic CT or USG remain credible imaging modalities for patients with suspected CDC.77
Patients with sinus infectionIt is important to emphasize that CT imaging tests cannot demonstrate the paranasal sinuses involvement in patients with early-stage Aspergillus rhinosinusitis, whose disease is restricted to the nasal cavity.
Acute Aspergillus infection of the paranasal sinuses appears in CT images as nonspecific dense opacification of the airspaces. Bone erosion is often absent or present only at late stage of infection and should not be considered a necessary finding for early diagnosis of acute Aspergillus sinusitis. Ethmoidal sinusitis with involvement of an orbit can occur in the absence of bony erosion of the lamina papyracea. Also consistent with this observation is that ethmoidal sinus aspergillosis can involve the medial rectus muscle of the orbit while the lamina papyracea remains intact. Increased diameter on a CT scan and elevated signal intensity on T2-weighted imaging will delineate the medial rectus infection. Similarly, aspergillosis of the frontal sinuses can be associated with infection of the frontal lobes without erosion of the walls of the frontal bone. Maxillary sinus aspergillosis can invade the palatine vessels and result in necrosis of the hard palate.66,78
The edema signal of the paranasal sinuses mucosa corresponds to the STIR hypersignal and T1 hyposignal. When this signal is modified to STIR hyposignal and T1 hypersignal (heavy fungi material), it is diagnostic of IFD. The sign of the fat of the posterior wall of maxillary sinus should also to be evaluated to characterize the extent of the disease.79
Patients with CNS infectionIn the pediatric cancer population, Aspergillus spp may infect the CNS via hematogenous dissemination to the brain parenchyma, seeding of CSF, or by direct extension from a sinus fungal infection, resulting in a clinical presentation that includes cerebritis, abscess, meningitis, and ventriculitis.80,81
Patterns of CNS aspergillosis identified in neuroimaging in immunocompromised patients include (1) multiple areas of hypodensity (CT) or hyperintensity (T2-weighted MRI) in the cortex and/or subcortical white matter corresponding to thromboembolic infarction with or without hemorrhage; (2) multiple intracerebral ring-enhancing lesions consistent with abscesses with a low signal (T2-weighted MRI); and (3) dural enhancement associated with enhancing lesions in the adjacent paranasal sinus structure or calvaria or dural enhancement of the optic sheath with associated optic nerve and intraorbital fat enhancement.82
In a recent review of 29 children submitted to hematologic stem cell transplantation who developed IFD by mold, it was found that 20% of the patients did not present any neurological symptoms at the time imaging results suggested the involvement of CNS by the fungal infection. Notably, all patients in this series presented at least one site of fungal infection besides the CNS, mostly the lungs. Based on their experience the authors suggested that facing a severely immunocompromised patient with diagnosis of IMD at any site we should consider a diagnostic work up including images of CNS.83 Further studies are necessary to confirm if this strategy is really cost-effective in clinical practice.
Frequency of image controlThis is a highly controversial issue that should be considered on a case by case basis. For patients with pneumonia and sinusitis, if there is a clear clinical improvement of the children, a new CT scan should not be considered before two weeks of treatment. In case of clinical deterioration and failure of response, a new imaging may be considered at any time.84 We were not able to find robust data to support any general recommendation on specific times for imaging control of patients with fungal sinusitis or CNS infection.66,70,78,85 Once the diagnosis of hepatosplenic IFD has been established, patient should be submitted to sequential imaging (every 30–60 days) to document complete resolution of the lesions. A new image and biochemical tests should be requested at any time in case of clinical deterioration.85,86
Strategies for diagnosing IFD in pediatric cancer patientsThe combination of different diagnostic tests is the best strategy for evaluating high-risk patients and results should be interpreted after considering a combination of variables: status of the underlying disease, exposure to risk factors, clinical presentation, availability of protecting environment for taking care of the patient, epidemiology of each medical center, as well as patient exposure to different regimens of antifungal prophylaxis or empirical therapy.44
In adults, GM, BDG and fungal PCR are the tools used to early diagnose IFD. However, only GM and BDG were incorporated into the criteria for definition of opportunistic IFD and invasive aspergillosis, according to EORTC/MSG.55
The use of these tests in pediatrics remains highly questionable as previously mentioned. A recent systematic review and meta-analysis addressing the use of fungal biomarkers in pediatric cancer and HSCT concluded that fungal biomarkers may present poor PPVs when used as screening tools during neutropenia or periods of GVHD.59 Otherwise, recently, Santolaya et al. showed good clinical results using GM in combination with imaging for driving antifungal therapy in pediatric patients with febrile neutropenia.61
According to data obtained with adult patients with hematologic malignancies, in case the clinician decides for early diagnostic-driven approach (preemptive antifungal therapy) for initiating antifungal therapy, a sequential collection of serum samples (at least twice a week) for detecting GM in high-risk patients for IFD is recommended to check for early diagnosis of IA.10 Otherwise, there is a lack of data supporting the efficacy of this strategy in pediatric cancer patients.10
In the absence of good scientific evidence to support the preemptive antifungal approach in pediatric patients, the latest guidelines for febrile neutropenia in children with cancer recommend the combination of imaging and fungal biomarkers only to investigate high risk pediatric patients with signs, symptoms and/or clinical instability.60
Of note, in non-neutropenic patients, in view of the limited sensitivity of serum GM for diagnosing aspergillosis, a negative result of this biomarker will not rule out the possibility of the disease. In this particular setting, it is better to consider the detection of GM in BAL sample where the sensitivity of the test increases to at least 80%.59
Disease-defining criteria according to EORTC-MSG (European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and by the National Institute of Allergy and Infectious Diseases Mycoses Study Group)
The definition of a case of IFD is based on a combination of evidences involving host factors, clinical data and mycological criteria. The main host factors to be considered are the presence of neutropenia, exposure to allogeneic stem cell transplant, treatment with high-dose corticosteroids and/or T cell immunosuppressants. The clinical criteria rely mostly on images of lower respiratory tract fungal disease (nodules, presence of halo sign, air-crescent sign or cavity on CT scan), sinonasal and CNS infection. The mycological criteria are based on the presence of fungal elements on direct microscopy or cytology examination, isolation of fungal pathogen in culture of normally sterile biologic samples, histopathology of tissue samples, and positive fungal biomarkers as galactomannan antigen detected in serum or in bronchoalveolar lavage fluid.55
The certainty categories of diagnosis are defined as possible, probable and proven, pending on the kind of evidence supporting the diagnosis. Probable IFD requires the presence of a host factor, a clinical criterion, and a mycological criterion not related to histopathology of the infected organ. Cases that meet the criteria for a host factor and a clinical criterion but for which mycological criteria are absent are considered possible IFD. The criteria for proven IFD includes culture from sterile material or histopathologic study of a specimen obtained by needle aspiration or biopsy in which hyphae are seen accompanied by evidence of associated tissue damage. Several series of studies conducted in adult cancer patients have suggested that a large proportion of cases classified as "probable" are confirmed by diagnosis after death, unlike the "possible" cases that are mostly other diseases.86,87
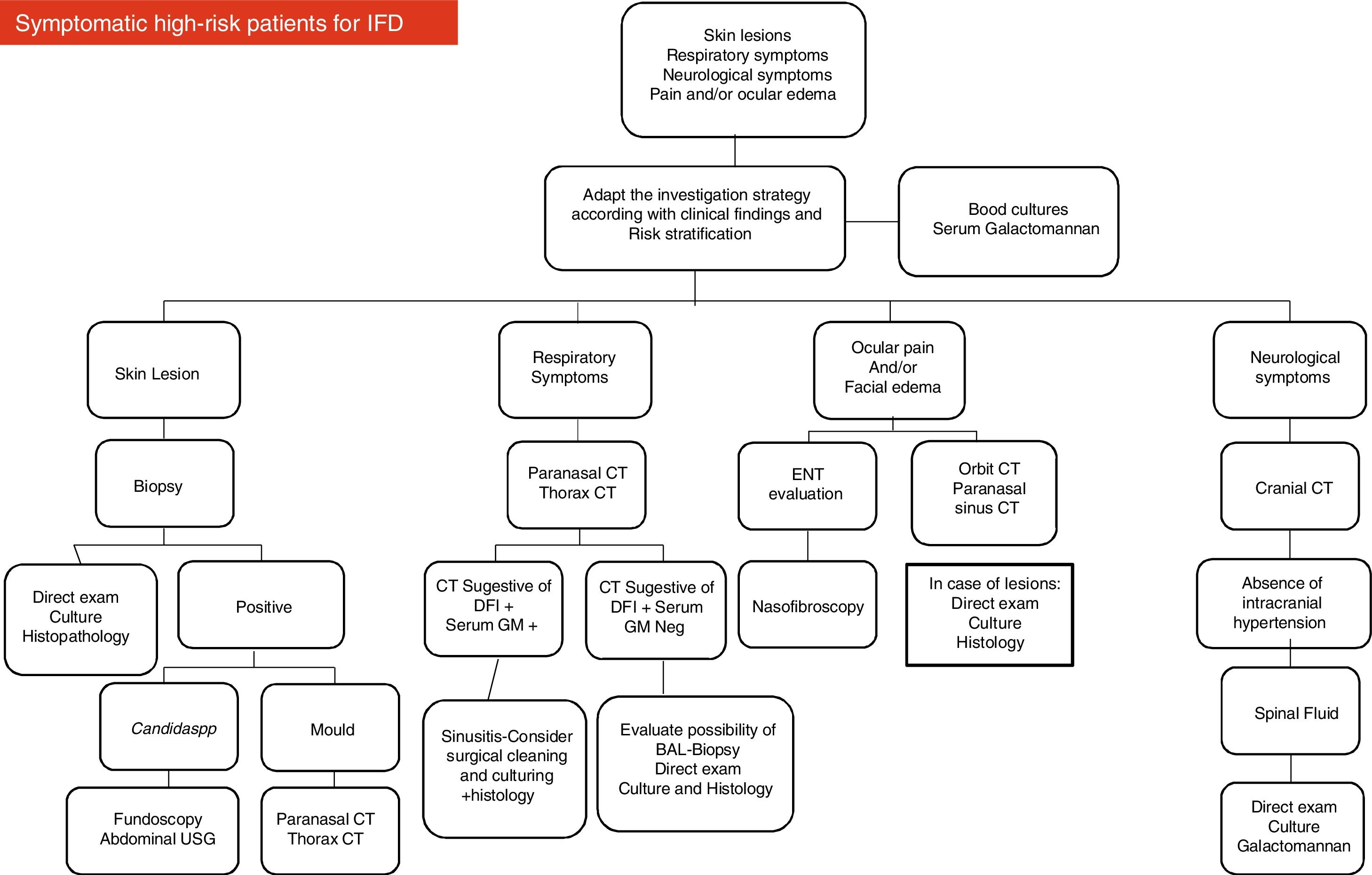
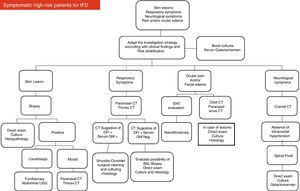
Based on their experience and data available in the medical literature, the present panel of specialists organized two diagrams and algorithms for the clinical management of pediatric oncology patients under high risk for developing IFD (see Figs. 1 and 2).
Notably, candidemia is usually documented by the presence of a positive blood culture. Fusariosis is usually documented by the presence of the pathogen in blood culture (sensitivity close to 60%), direct examination and culture of skin lesions of material from the respiratory tract. Mucormycosis is usually documented by the presence of large and non-septate hyphae in cytology or biopsy of infected tissue samples once cultures have low sensitivity and fungal biomarkers are usually negative. Aspergillosis is usually detected by the combination of GM in serum and/or BAL sample of high-risk patients exhibiting images compatible with fungal involvement in respiratory tract.44
Different strategies for antifungal therapy of IFDAntifungal prophylaxisProphylaxis against invasive Candida infections with fluconazole is recommended for all pediatric patients with acute myeloid leukemia, recurrent acute leucemia, allogeneic HSCT and high-risk acute lymphoblastic leukemia. Local epidemiology and patient-specific risk factors, such as comorbidity or specific treatment modalities, are important considerations for the choice of an appropriate antifungal prophylactic strategy.10
Recent guidelines recommend mould-active antifungal prophylaxis, mainly with new triazoles, for high risk hematological malignancies and HSCT transplant recipients.84,85 Posaconazole prophylaxis can be used for patients over 13 years old.88 In general, anti-mould prophylaxis are only cost-effective in patients with incidence rates of invasive mould infections higher than 7–10%. Otherwise, it increases costs, toxicity and the risk of antifungal resistance.1,10,85
Empiric antifungal therapy versus diagnostic-driven antifungal treatmentEmpirical antifungal therapy has been a common practice in granulocytopenic children with persistent fever despite four days of appropriate empirical antibacterial therapy. This strategy should be considered only for high-risk granulocytopenic children with newly diagnosed or recurrent acute leukemia and in those undergoing allogeneic HSCT. Antifungal therapy should be used up to the resolution of granulocytopenia in spite of the absence of documented IFD. All efforts must be done to elucidate the diagnosis in order to reduce unnecessary exposure of patients to antifungal therapy.10
Multiple studies conducted in adult population indicate that monitoring serum GM and Aspergillus-PCR in patients with acute leukemia or myelodysplasia and HSCT recipients during neutropenia is useful for the early diagnosis of IA. When combined with aggressive CT scanning of sinuses and lungs, this strategy may safely replace the conventional regimen of empirical antifungal therapy driven by febrile neutropenia refractory to antibiotics in cancer patients.53,70,85,89,90 Unfortunately, it is still unclear if this strategy may be successfully extrapolated for pediatric cancer patients.
Recently, Santolaya et al. performed a prospective multicenter randomized trial in Santiago, Chile. Children presenting with persistent high-risk FN were randomized to empirical or pre-emptive antifungal therapy. They included 149 children, 73 received empirical therapy and 76 pre-emptive therapy. Only 32/76 (42%) received antifungal therapy in this group. The overall mortality was similar in both groups, IFD mortality was the same (3%); pre-emptive therapy was as effective as empirical antifungal therapy in these children.61 This was the first prospective, multicenter, randomized study comparing standard versus pre-emptive antifungal therapy in HRFN children with prolonged fever and neutropenia. More studies performed with pediatric patients are necessary before we establish any robust recommendation for the routine use of pre-emptive antifungal therapy in this setting.
Antifungal therapy of patients with documented fungal infectionsInvasive aspergillosisVoriconazole has been considered by several guidelines as the treatment of choice for this condition, but monitoring of drug plasma levels is recommended to optimize antifungal therapy.10,84,85,91 In order to avoid toxicity and optimize clinical results, the target plasmatic concentration of voriconazole should remain is the range of 1–5 mg/L.92 Liposomal amphotericin B, initially at 3 mg/kg/day, is an acceptable alternative for patients aged less than two years old, as well as facing any contraindication for voriconazole use.10
There is no evidence to support the use of combination antifungal therapy for treating children with aspergillosis. Recently, Isavuconazole was found to be equally efficient and less toxic than voriconazole in a randomized clinical trial performed with adult patients.93 So far, there are no clinical studies addressing the use of isavuconzole in children with IA.
Invasive fusariosisThe genus Fusarium contains many species and is widely distributed in nature. Human fusariosis manifests as superficial infections (dermatomycosis and onychomycosis), localized subcutaneous infections and, in severely immunosuppressed patients, sinus, lung or disseminated disease.40,94 Therapeutic outcomes are poor due to underlying severe immunosuppression and suboptimal susceptibility of the pathogen to almost all antifungals. The European Society of Clinical Microbiology and Infectious Diseases/European Confederation of Medical Mycology guidelines for treatment of rare mould infections recommends the use of voriconazole as the best alternative for fusariosis, being lipid formulations of AMB accepted as alternative choices.95,96 Considering the concerns with voriconazole plasma levels in children, combination therapy of amphotericin B lipid formulation with voriconazole has been extensively used by several medical centers.42,43,94
Invasive mucormycosisThe optimal treatment of mucormycosis has not been defined, due to the lack of appropriate prospective and randomized clinical trials. Amphotericin B deoxycholate (AmB-d) at maximum tolerable doses (1–1.5 mg/kg/day) was historically the antifungal treatment of choice prior to the availability of less nephrotoxic lipid formulations of amphotericin B. Lipid formulations of AMB, starting at 5 mg/kg/day, are now preferred as first-line therapy, with a higher initial dose of 10 mg/kg/day recommended in cases of CNS disease by some investigators.97
Based on observational data, posaconazole may have a role in patients who require ongoing maintenance therapy.97 Maintenance therapy is indicated in patients with residual tissue-based infection or with risk factors that are not readily modifiable. In these patients, therapeutic drug monitoring should be performed where possible. Given the paucity of high-quality evidence and the uncertain efficacy and safety of this strategy, combination polyene-echinocandin therapy cannot currently be recommended. Similar to adults, surgery combined with antifungal therapy is a major factor to increase survival.97
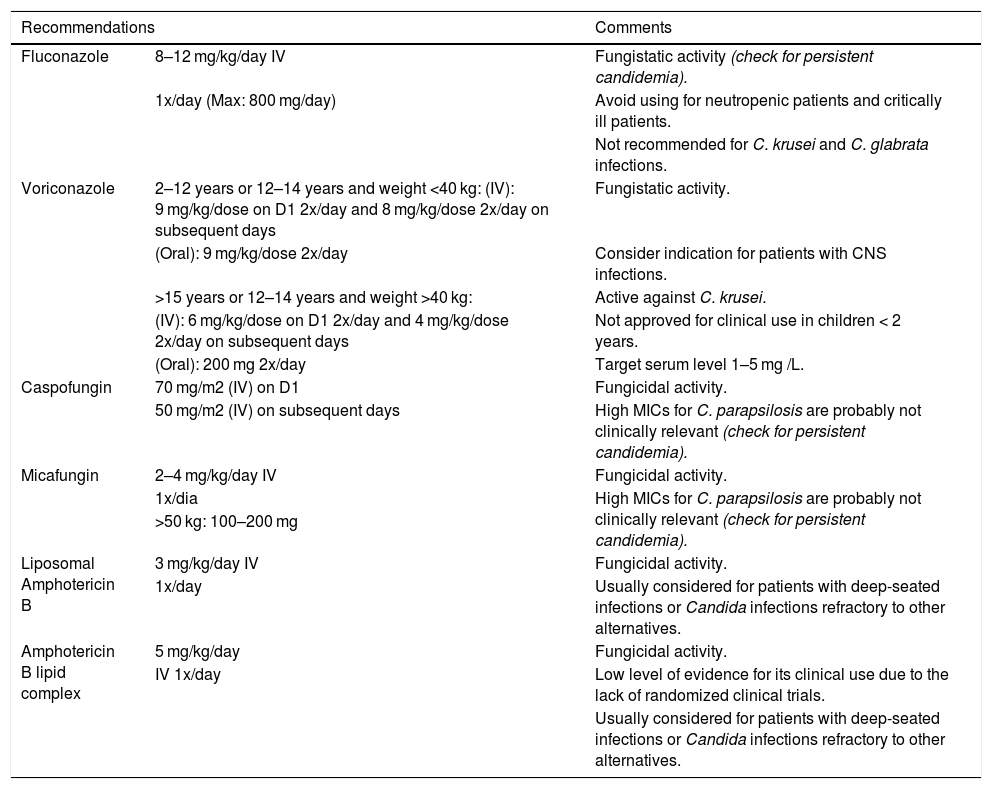
Invasive candidiasisPatients should be initially treated with echinocandins as suggested by most guidelines published recently.98–101 Considering the lack of data on the safety and efficacy of anidulafungin in pediatric patients, caspofungin and micafungin represent the most appropriate options for children among the echinocandins. Formulations of AMB are also considered as alternative, especially in patients with CNS infections or endocarditis.99–101
After initial course of therapy with broad spectrum antifungals, step-down therapy with fluconazole may be considered after clinical stabilization and facing the correct identification of the pathogen. Treatment with fluconazole is not recommended for infections by Candida krusei and Candida glabrata, since C. krusei is inherently resistant, and C. glabrata has low susceptibility to this agent (Tables 2–5).99,100
Treatment of Invasive Candidiasis (adapted from Groll 2014 and Colombo-2013).10,101
| Recommendations | Comments | |
|---|---|---|
| Fluconazole | 8–12 mg/kg/day IV | Fungistatic activity (check for persistent candidemia). |
| 1x/day (Max: 800 mg/day) | Avoid using for neutropenic patients and critically ill patients. | |
| Not recommended for C. krusei and C. glabrata infections. | ||
| Voriconazole | 2–12 years or 12–14 years and weight <40 kg: (IV): 9 mg/kg/dose on D1 2x/day and 8 mg/kg/dose 2x/day on subsequent days | Fungistatic activity. |
| (Oral): 9 mg/kg/dose 2x/day | Consider indication for patients with CNS infections. | |
| >15 years or 12–14 years and weight >40 kg: | Active against C. krusei. | |
| (IV): 6 mg/kg/dose on D1 2x/day and 4 mg/kg/dose 2x/day on subsequent days | Not approved for clinical use in children < 2 years. | |
| (Oral): 200 mg 2x/day | Target serum level 1–5 mg /L. | |
| Caspofungin | 70 mg/m2 (IV) on D1 | Fungicidal activity. |
| 50 mg/m2 (IV) on subsequent days | High MICs for C. parapsilosis are probably not clinically relevant (check for persistent candidemia). | |
| Micafungin | 2–4 mg/kg/day IV | Fungicidal activity. |
| 1x/dia | High MICs for C. parapsilosis are probably not clinically relevant (check for persistent candidemia). | |
| >50 kg: 100–200 mg | ||
| Liposomal Amphotericin B | 3 mg/kg/day IV | Fungicidal activity. |
| 1x/day | Usually considered for patients with deep-seated infections or Candida infections refractory to other alternatives. | |
| Amphotericin B lipid complex | 5 mg/kg/day | Fungicidal activity. |
| IV 1x/day | Low level of evidence for its clinical use due to the lack of randomized clinical trials. | |
| Usually considered for patients with deep-seated infections or Candida infections refractory to other alternatives. | ||
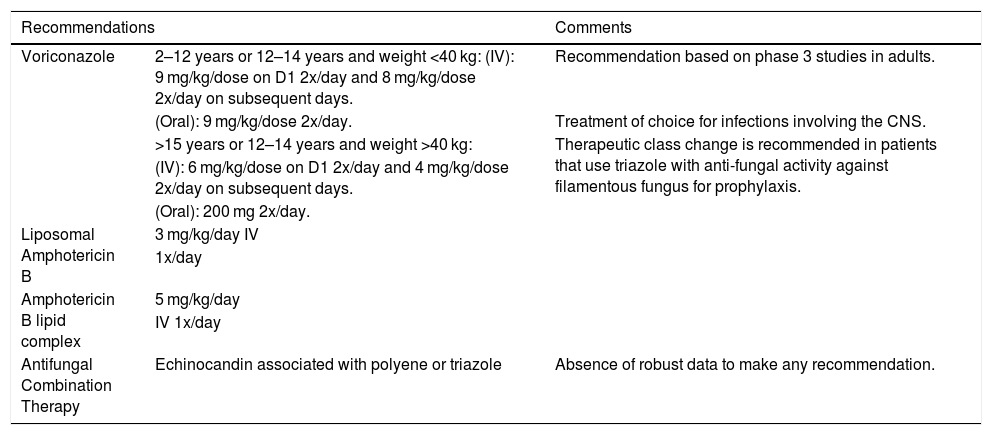
Treatment of Invasive Aspergilosis (adapted from Groll et al., 2014).10
| Recommendations | Comments | |
|---|---|---|
| Voriconazole | 2–12 years or 12–14 years and weight <40 kg: (IV): 9 mg/kg/dose on D1 2x/day and 8 mg/kg/dose 2x/day on subsequent days. | Recommendation based on phase 3 studies in adults. |
| (Oral): 9 mg/kg/dose 2x/day. | Treatment of choice for infections involving the CNS. | |
| >15 years or 12–14 years and weight >40 kg: | Therapeutic class change is recommended in patients that use triazole with anti-fungal activity against filamentous fungus for prophylaxis. | |
| (IV): 6 mg/kg/dose on D1 2x/day and 4 mg/kg/dose 2x/day on subsequent days. | ||
| (Oral): 200 mg 2x/day. | ||
| Liposomal Amphotericin B | 3 mg/kg/day IV | |
| 1x/day | ||
| Amphotericin B lipid complex | 5 mg/kg/day | |
| IV 1x/day | ||
| Antifungal Combination Therapy | Echinocandin associated with polyene or triazole | Absence of robust data to make any recommendation. |
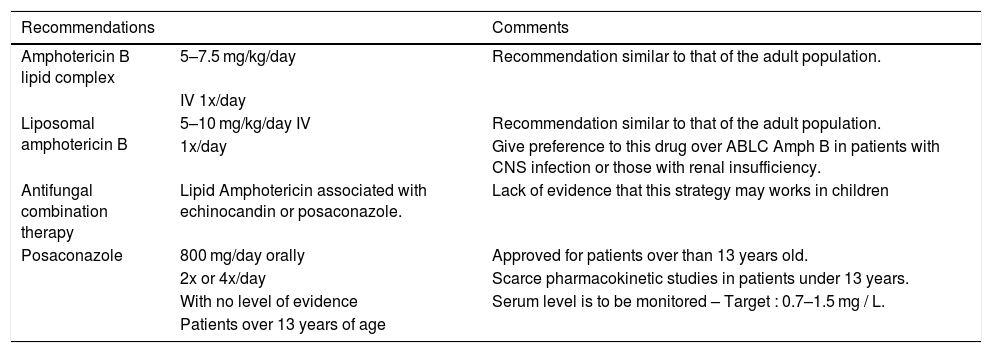
Treatment of Invasive Mucormycosis (adapted from Groll, 2014).10
| Recommendations | Comments | |
|---|---|---|
| Amphotericin B lipid complex | 5–7.5 mg/kg/day | Recommendation similar to that of the adult population. |
| IV 1x/day | ||
| Liposomal amphotericin B | 5–10 mg/kg/day IV | Recommendation similar to that of the adult population. |
| 1x/day | Give preference to this drug over ABLC Amph B in patients with CNS infection or those with renal insufficiency. | |
| Antifungal combination therapy | Lipid Amphotericin associated with echinocandin or posaconazole. | Lack of evidence that this strategy may works in children |
| Posaconazole | 800 mg/day orally | Approved for patients over than 13 years old. |
| 2x or 4x/day | Scarce pharmacokinetic studies in patients under 13 years. | |
| With no level of evidence | Serum level is to be monitored – Target : 0.7–1.5 mg / L. | |
| Patients over 13 years of age | ||
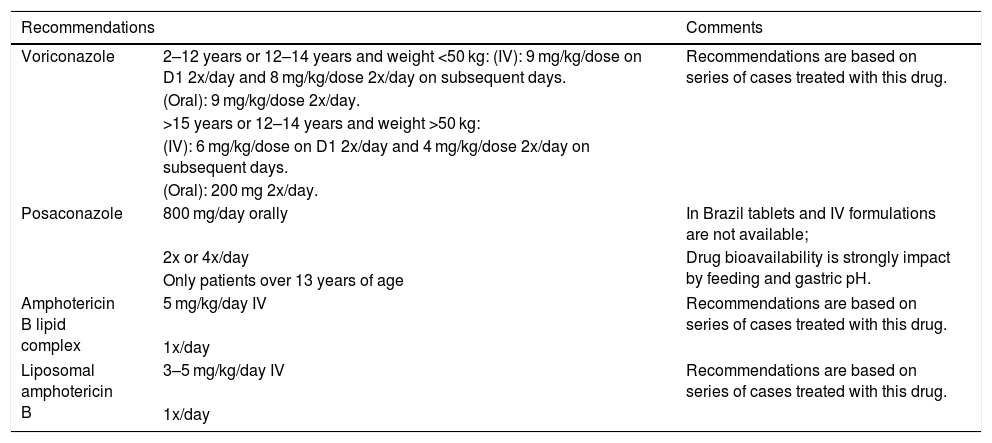
Treatment of Invasive Fusariosis (adapted from Groll, 2014).10
| Recommendations | Comments | |
|---|---|---|
| Voriconazole | 2–12 years or 12–14 years and weight <50 kg: (IV): 9 mg/kg/dose on D1 2x/day and 8 mg/kg/dose 2x/day on subsequent days. | Recommendations are based on series of cases treated with this drug. |
| (Oral): 9 mg/kg/dose 2x/day. | ||
| >15 years or 12–14 years and weight >50 kg: | ||
| (IV): 6 mg/kg/dose on D1 2x/day and 4 mg/kg/dose 2x/day on subsequent days. | ||
| (Oral): 200 mg 2x/day. | ||
| Posaconazole | 800 mg/day orally | In Brazil tablets and IV formulations are not available; |
| 2x or 4x/day | Drug bioavailability is strongly impact by feeding and gastric pH. | |
| Only patients over 13 years of age | ||
| Amphotericin B lipid complex | 5 mg/kg/day IV | Recommendations are based on series of cases treated with this drug. |
| 1x/day | ||
| Liposomal amphotericin B | 3–5 mg/kg/day IV | Recommendations are based on series of cases treated with this drug. |
| 1x/day | ||
Patients with hepatosplenic candidiasis usually requires several weeks or months of therapy what usually demands induction with echinocandins followed by long periods of fluconazole up to complete resolution of lesions.99–101
Conflicts of interestThe authors declare no conflicts of interest.